
UC Irvine
UC Irvine Previously Published Works
Title
Segregation of two endocannabinoid‐hydrolyzing enzymes into pre‐ and postsynaptic
compartments in the rat hippocampus, cerebellum and amygdala
Permalink
https://escholarship.org/uc/item/86z7c238
Journal
European Journal of Neuroscience, 20(2)
ISSN
0953-816X
Authors
Gulyas, AI
Cravatt, BF
Bracey, MH
et al.
Publication Date
2004-07-01
DOI
10.1111/j.1460-9568.2004.03428.x
Copyright Information
This work is made available under the terms of a Creative Commons Attribution License,
availalbe at https://creativecommons.org/licenses/by/4.0/
Peer reviewed
eScholarship.org Powered by the California Digital Library
University of California

Segregation of two endocannabinoid-hydrolyzing
enzymes into pre- and postsynaptic compartments
in the rat hippocampus, cerebellum and amygdala
A. I. Gulyas,
1
B. F. Cravatt,
2
M. H. Bracey,
2
T. P. Dinh,
3
D. Piomelli,
3
F. Boscia
4
and T. F. Freund
1
1
Institute of Experimental Medicine, Hungarian Academy of Sciences, Budapest, PO Box 67, H-1450, Hungary
2
The Scripps Research Institute, 10550 N. Torrey Pines Road, La Jolla, CA 92037, USA
3
Department of Pharmacology, University of California Irvine, Irvine, CA 92697, USA
4
Department of Neuroscience, Section of Pharmacology, School of Medicine, University of Naples ’Federico II’, Via Pansini 5,
80131-Naples, Italy
Keywords: 2-AG, anandamide, Ca
2+
stores, electron microscopy, inhibitory cells, interneurons
Abstract
Fatty acid amide hydrolase (FAAH) and monoglyceride lipase (MGL) catalyse the hydrolysis of the endocannabinoids anandamide
and 2-arachidonoyl glycerol. We investigated their ultrastructural distribution in brain areas where the localization and effects of
cannabinoid receptor activation are known. In the hippocampus, FAAH was present in somata and dendrites of principal cells, but not
in interneurons. It was located mostly on the membrane surface of intracellular organelles known to store Ca
2+
(e.g. mitochondria,
smooth endoplasmic reticulum), less frequently on the somatic or dendritic plasma membrane. MGL immunoreactivity was found in
axon terminals of granule cells, CA3 pyramidal cells and some interneurons. In the cerebellum, Purkinje cells and their dendrites are
intensively immunoreactive for FAAH, together with a sparse axon plexus at the border of the Purkinje cell ⁄ granule cell layers.
Immunostaining for MGL was complementary, the axons in the molecular layer were intensively labelled leaving the Purkinje cell
dendrites blank. FAAH distribution in the amygdala was similar to that of the CB
1
cannabinoid receptor: evident signal in neuronal
somata and proximal dendrites in the basolateral nucleus, and hardly any labelling in the central nucleus. MGL staining was restricted
to axons in the neuropil, with similar relative signal intensities seen for FAAH in different nuclei. Thus, FAAH is primarily a
postsynaptic enzyme, whereas MGL is presynaptic. FAAH is associated with membranes of cytoplasmic organelles. The differential
compartmentalization of the two enzymes suggests that anandamide and 2-AG signalling may subserve functional roles that are
spatially segregated at least at the stage of metabolism.
Introduction
The identity and localization of cannabinoid receptors as well as their
endogenous ligands have been recently reported (Devane et al.,
1988,1992; Matsuda et al., 1990; Stella et al., 1997). In the
hippocampus and cerebellum, cannabinoid receptors subtype 1
(CB
1
) were shown to mediate depolarization-induced suppression of
inhibition and excitation (Kreitzer & Regehr, 2001a,b; Wilson &
Nicoll, 2001; Ohno-Shosaku et al., 2001), suggesting that endocann-
abinoids act as retrograde modulators of synaptic signalling.
Activity-dependent release of endocannabinoids from hippocampal
pyramidal and cerebellar Purkinje cells activate cannabinoid receptors
located on excitatory and inhibitory axon terminals (Katona et al.,
1999), and reduce c-aminobutyric acid (GABA; Hajos et al., 2000;
Hoffman & Lupica, 2000) and glutamate (Shen et al., 1996; Misner &
Sullivan, 1999) release. Recent experiments suggest that retrograde
modulation of glutamatergic and GABAergic transmission may
involve different cannabinoid receptors. In the hippocampus, CB
1
is
selectively located in GABAergic axons (Katona et al., 1999),
whereas glutamate release is regulated by a so far unidentified receptor
(Hajos et al., 2001).
The two well-established endogenous cannabinoids are anandamide
(the ethanolamide of arachidonic acid) and sn-2-arachidonoyl-glycerol
(2-AG). A phospholipase D ⁄ N-acetyltransferase-dependent pathway
is responsible for the synthesis of anandamide (Cadas et al., 1997),
while phospholipase C and diacylglycerol lipase are thought to be
involved in the synthesis of 2-AG (Stella et al., 1997). Electrical
stimulation of hippocampal slices increases the levels of 2-AG (Stella
et al., 1997). In cultured cortical neurons, activation of N-methyl-d-
aspartate receptors increases 2-AG levels but has no effect on
anandamide formation, which requires instead the simultaneous
activation of N-methyl-d-aspartate and a-7 nicotinic receptors (Stella
& Piomelli, 2001).
Fatty acid amide hydrolase (FAAH) and monoglyceride lipase
(MGL) have been identified as degrading enzymes of endogenous
cannabinoids. Breakdown of 2-AG has been attributed to MGL (Dinh
et al., 2002). In contrast, the experiments demonstrating that FAAH – ⁄ –
mutant mice cannot metabolize anandamide (Cravatt et al., 2001)
while 2-AG hydrolysis is preserved (Lichtman et al., 2002) suggest
that FAAH is the main anandamide-metabolizing enzyme.
The distribution of CB
1
receptors in different areas of the brain
has been outlined using radioligand binding (Herkenham et al.,
Correspondence: Dr A. I. Gulyas, as above.
E-mail: [email protected]u
Received 19 March 2004, revised 7 April 2004, accepted 13 April 2004
European Journal of Neuroscience, Vol. 20, pp. 441–458, 2004 ª Federation of European Neuroscience Societies
doi:10.1111/j.1460-9568.2004.03428.x
1990; Mailleux & Vanderhaeghen, 1992). The receptor is expressed
in several brain areas with a characteristic pattern, often associated
with identified cell types. However, no data are available yet on the
cellular and subcellular localization of the enzymes that catalyse
endocannabinoid hydrolysis, except for low-resolution light micro-
scopic descriptions of FAAH (Egertova et al., 2003) and MGL
(Dinh et al., 2002). The brain distribution of FAAH and MGL
mRNA have been reported (Thomas et al., 1997; Dinh et al., 2002).
Identifying the exact sites of elimination of anandamide and 2-AG
may shed light on their functional roles and help to understand their
mechanisms of deactivation in the intact brain. Therefore, the
present study investigated the distribution of FAAH and MGL at the
light and electron microscopic levels in three brain regions in which
endocannabinoid signalling has been shown to play important roles:
the hippocampus, amygdala and cerebellum. The presence of the
enzymes in different marker-containing, functionally distinct inter-
neuron types has also been studied in the hippocampus using
double-labelling methods.
Materials and methods
Handling and perfusion of animals
Experiments were performed according to the guidelines of the
Institutional Ethical Codex & the Hungarian Act of Animal Care &
Experimentation (1998, XXVIII, Section 243 ⁄ 1998), which is in full
agreement with the regulation of animal experiments in the European
Union. All efforts were made to reduce the number of animals used.
For the localization of FAAH, 13 adult (250 g) male Wistar rats
(Charles-River, Hungary) and four adult mice (two FAAH + ⁄ + and
two FAAH – ⁄ –) were perfused under equithesine anaesthesia
(chlornembutal 0.3 mL ⁄ 100 g), first with physiological saline
(1 min) and then with a fixative containing 1% glutaraldehyde
(TAAB, UK), 3% paraformaldehyde (TAAB, UK) and 0.05% picric
acid in 0.1 m phosphate buffer (PB) for 30 min. For the MGL
immunostainings, six rats were perfused with the fixative described
above.
After fixation, the dorsal hippocampi were dissected and sectioned
on a vibratome at 60 lm. Following extensive washes in PB, the
sections were immersed in a mixture of 25% sucrose and 10% glycerol
in 0.1 m PB, and freeze-thawed over liquid nitrogen to increase the
penetration of antisera.
Pre-embedding immunostaining
Sections were washed three times for 30 min between each step. All
the washing steps and the dilution of the antisera were carried out in
50 mm Tris-buffered saline (pH 7.4). The sections were incubated
first in 2% bovine serum albumin (for 45 min, Sigma), then in one
of the primary antibodies for 2 days at 4. Rabbit anti-FAAH
antisera were generated against FAAH-GST (glutathione S-transfer-
ase) fusion protein (Patricelli et al., 1998; anti-FAAH, used in
1 : 1500), the other directed against a native, 6X-His tagged
truncation of FAAH purified as described in Bracey et al. (2002;
anti-FAAH-DTM, used in 1 : 3000). The rabbit anti-MGL serum
(Dinh et al., 2002) was used in 1 : 5000 dilution. Following the
primary antisera, sections for immunoperoxidase reaction were
incubated in biotinylated goat anti-rabbit IgG (1 : 300 Vector
Laboratories, CA, USA, 4 h) and then in Elite ABC (1 : 400
Vector Laboratories, 3 h). The peroxidase reaction was developed by
3,3¢-diaminobenzidine)4HCl (DAB, Fluka Sigma-Aldrich, Hungary)
as a chromogen. After the final washes in PB, the sections were
treated with 1% OsO
4
for 1 h, dehydrated in ethanol and embedded
in Durcupan (Fluka Sigma-Aldrich).
For pre-embedding immunogold staining against FAAH, the
sections were incubated in anti-FAAH-DTM antiserum (1 : 500,
2 days) followed by 1 nm gold conjugated goat anti-rabbit antibody
(1 : 50, overnight incubation, Amersham, UK). Gold labelling was
intensified using the R-Gent silver intensification kit (Aurion,
Wageningen, the Netherlands). Sections were then osmicated (0.5%
OsO
4
, 30 min, 4 C), dehydrated and embedded in Durcupan.
Double pre-embedding immunostaining
We aimed to study the co-localization of MGL and cholecystokinin
(CCK) at the ultrastructural level. For this purpose, the following
staining procedure was carried out: the sections were incubated in a
mixture of rabbit anti-MGL (1 : 5000) and mouse anti-CCK
(1 : 2000, CURE, Digestive Diseases Research Center, USA)
antibodies for 2 days. This was followed by incubation in gold
conjugated goat anti-mouse (1 : 50, overnight incubation, Aurion)
and silver intensification (see above) of the gold particles to detect
CCK. Biotinylated goat anti-rabbit IgG (1 : 300, 4 h, Vector
Laboratories) and Elite ABC (1 : 400, 3 h, Vector Laboratories),
followed by developing of the reaction with DAB was used to
visualize MGL.
Double-immunofluorescent staining
Incubation of sections in 2% bovine serum albumin (see above) was
followed by mixtures of primary antibodies for overnight incubation:
rabbit anti-FAAH-DTM (1 : 500) was mixed with mouse anti-GABA
(1 : 75, Szabat et al., 1992) or mouse anti-GAD65 (1 : 200,
CHEMICON International, Temecula, USA) or mouse anti-parvalbu-
min (PV; 1 : 1000, Fluka Sigma-Aldrich) or mouse anti-calbindin
(1 : 6000, Fluka Sigma-Aldrich) or mouse anti-CCK (1 : 5000,
CURE, Digestive Diseases Research Center) or mouse anti-calretinin
(1 : 1000, SWANT, Bellinzona, Switzerland). After repeated washes
in Tris-buffered saline, the sections were incubated in mixtures of
fluorescent-labelled secondary antibodies for 2 h. Against the FAAH
antibody we used goat anti-rabbit-FITC (1 : 50, Jackson Immuno-
Research Laboratories, Pennsylvania, USA), that was mixed with goat
anti-mouse-Cy3 (1 : 200, Jackson ImmunoResearch Laboratories) to
label the other markers. The sections were then washed in Tris-
buffered saline, transferred onto microscope slides and covered with
Vectashield (Vector Laboratories). The sections were evaluated using a
Zeiss (Germany) Axioplan2 microscope with filters for FITC (exci-
tation BP450–490, emission BP515–565) and for Cy3 (excitation
BP546 ⁄ 12, emission LP590).
Controls
Antisera specificity was confirmed by the laboratories of origin
(Patricelli et al., 1998; Bracey et al., 2002; Dinh et al., 2002). Controls
of the methods in the present experiments included replacement of the
primary antisera with normal serum (1 : 200). In the case of the FAAH
antibodies, specificity was verified by the fact that no staining could be
seen in the FAAH-KO animals. In case of MGL the antibody was
preabsorbed with the immunizing peptide (SSPRRTPQNVPYQDL).
In the latter cases no signal was visible apart from a faint background
limited to the surface of the sections. In double-labelled sections the
pattern of immunoreactivity for both antigens was identical to that
seen in single-stained material.
442 A. I. Gulyas et al.

Quantitative analysis of subcellular distribution of FAAH
To ensure an unbiased estimation of the relative distribution of silver-
intensified immunogold particles over different cellular compartments,
a random sampling was made in selected areas of the hippocampus
and cerebellum. High-magnification non-overlapping electron micro-
graphs were taken randomly, using a MegaViev II CCD camera, from
the following areas: hippocampus CA1 area str. radiatum, cerebellum
str. moleculare, cerebellum Purkinje cells bodies. Because we wanted
to know the relative distribution of gold particles among different
compartments and the size of the gold particles is small compared to
the area of the electron micrographs, no special stereological sampling
method had to be used. For the counting we identified the position of
each gold particle over (or next to) the following elements: cell surface
membrane, endoplasmic reticulum, mitochondrion outer membrane,
stacked saccules of smooth endoplasmic reticulum (in the cerebellum
only) and cytoplasm. A particle was assigned to one of the membrane-
delineated compartments if the particle was within 40 nm from a
membrane (we chose this value because it is the average diameter of a
silver-intensified gold particle). If a particle was further away it was
assigned to the cytoplasm.
Results
Two antibodies were used to visualize the distribution of FAAH at the
cellular and subcellular levels. Anti-FAAH was raised against FAAH-
GST fusion protein, while the anti-FAAH-DTM was raised against a
native, 6X-His tagged truncation of FAAH. Staining with the two
antibodies resulted in identical labelling pattern, as shown in Fig. 1 in
the hippocampus. The specificity of the FAAH antibodies was verified
by immunostaining hippocampal sections from FAAH-KO mice. No
signal could be detected with either of the antibodies (Fig. 1C and D).
Because the anti-FAAH-DTM antibody worked best at a higher
dilution than the anti-FAAH and gave a signal with somewhat lower
background, in the rest of the study we used the anti-FAAH-DTM for
immunostaining at the light and electron microscopic levels. In the
case of FAAH both the DAB and the pre-embedding gold visualiza-
tion methods were employed for precise subcellular localization of
the protein. In the case of MGL, ultrastructural localization was
investigated with DAB alone. Immunogold localization was not
required as, opposed to FAAH, MGL is a cytosolic protein (Dinh
et al., 2002).
Light microscopical distribution of FAAH in the hippocampus
The distribution of FAAH in pyramidal cells of the hippocampus has
been described recently at the light microscopic level (Tsou et al.,
1998b; Egertova et al., 2003) using the same antisera. The present
study confirmed these results, which will be described only briefly.
Attention will be focused on our novel observations, namely the
presence of FAAH immunoreactivity in different interneuron types
and the subcellular distribution of FAAH at the electron microscopic
level using immunogold labelling.
It is evident already at low magnification (Fig. 1A and B) that
FAAH is associated with principal neurons of the hippocampus, as the
principal cell layers are distinctively immunoreactive. At higher
magnification a fine-grained, reticular immunoperoxidase reaction
characterizes the immunostaining in the principal cell cytoplasm,
proximal dendrites, as well as in the neuropil. In the CA1 area, the
signal is present in all layers (Fig. 2A). The strongest signal is visible
in the cytoplasm of the pyramidal cells, no labelling can be detected in
the nucleus. Distinctly labelled by FAAH immunoreactivity, the apical
and basal pyramidal cell dendrites can be followed for considerable
distances. The intensity of the labelling decreases distally from str.
pyramidale, most probably due to pyramidal cell dendrites forming
thinner, secondary branches that are covered with dendritic spines. In
the distal two-thirds of str. oriens, the upper half of str. radiatum and
the str. lacunosum-moleculare, a fine-grained neuropil staining is
displayed. In the CA3 area the distribution of FAAH is very similar to
Compartmentalization of endocannabinoid hydrolysis 443
Fig. 1. Specificity of the fatty acid amide hydrolase (FAAH) antibodies used. Two antibodies were raised: one against a FAAH-GST fusion protein (anti-FAAH),
the other against 6X-His tagged truncation of FAAH (anti-FAAH-DTM). As shown in A and B, the two antibodies gave identical staining in the hippocampus of
wild-type (WT) mice. The specificity of the antibodies was demonstrated by
the complete lack of signal in sections deriving form FAAH KO mice (C and D). Scale
bar: 1 mm. Abbreviations: DG, dentate gyrus; hil., hilus; s.g., str. granulosum; s.m., str. moleculare; s.o., str. oriens; s.p., str. pyramidale; s.r., str. radiatum.

Fig. 2. FAAH is located in the principal cells of the hippocampus. (A–C) The somata and the dendrites (arrows) of the CA1 (A) and CA3 (B) pyramidal cells as well
as the granule cells of the dentate gyrus (C) are immunopositive for FAAH. In the hilus of the dentate gyrus the mossy cells (large arrows) are also expressing FAAH.
(D and E) Interneurons (arrowheads) can be identified as unstained elements in the FAAH-positive neuropil in str. oriens of the CA1 (D) and CA3 (E) areas. Some
FAAH-positive pyramidal cell bodies are labelled with arrows. Scale bars, 100 lm (A and B); 50 lm (C); 20 lm (D and E). Abbreviations: DG, dentate gyrus; hil., hilus;
s.g., str. granulosum; s.l., str. lucidum; s.l-m, str. lacunosum-moleculare; s.m., str. moleculare; s.o., str. oriens; s.p., str. pyramidale; s.r., str. radiatum.
444 A. I. Gulyas et al.

the CA1 area. The only exception is that, due to the FAAH negativity
of the numerous mossy fibres, the neuropil labelling is low in str.
lucidum (Fig. 2B). In the gyrus dentatus, FAAH signal in the somata
and dendrites of the granule cells is somewhat weaker than in the
pyramidal cells. However, the staining is intense in the hilus. As
shown in Fig. 2C, mossy cells express FAAH and the neuropil is also
labelled. In contrast to the principal cells, interneurons do not show
FAAH immunoreactivity. These cells can be identified as negative
islands in the FAAH immunoreactive neuropil in all areas and layers
(Fig. 2C and E).
To verify that FAAH is not present in inhibitory interneurons, we
made double-immunofluorescent localization against FAAH and
different neurochemical markers present in most (GAD65) or in
smaller, functionally distinct subpopulations of interneurons (for
Compartmentalization of endocannabinoid hydrolysis 445
Fig. 3. FAAH is not detectable in GABAergic interneurons. Double-immunofluorescent staining against FAAH (green) and different markers (red) present in
subpopulations of inhibitory interneurons. Arrowheads indicate interneurons negative for FAAH and positive for the given markers. FAAH-positive principal
neurons are labelled with small arrows. Small arrowheads in D indicate FAAH-positive pyramidal cell dendrites in CA1 str. radiatum. In G, double arrowheads label
CA1 pyramidal cells that are positive for both FAAH and calbindin D28k (CB). (A and C) Hilus of the dentate gyrus; (B, D, F and G) CA1 area; (E and H) CA3 area.
The following GABAergic neuronal markers were examined: GAD65, glutamic acid decarboxylase 65
kDa; CCK, cholecystokinin; PV, parvalbumin; CB, calbindin
D28k; CR, calretinin. Scale bars, 20 lm. Abbreviations: DG, dentate gyrus; hil., hilus; s.g., str. granulosum; s.l-m, str. lacunosum-moleculare; s.m., str. moleculare;
s.o., str. oriens; s.p., str. pyramidale; s.r., str. radiatum.

review, see Freund & Buzsaki, 1996), such as CCK, PV, calbindin
D28k (CB) and calretinin (CR). As shown in Fig. 3, none of the
marker-containing inhibitory neurons expressed FAAH. Co-localiza-
tion of two signals could only be seen in the case of CB labelling in
the CA1 superficial pyramidal cells and the dentate granule cells, as
these principal neurons express CB (Sloviter, 1989).
Ultrastructural localization of FAAH in the hippocampus
The electron microscopical localization of FAAH was studied both by
immunoperoxidase (DAB, Fig. 4A) and by pre-embedding immuno-
gold staining (Fig. 4B–D). The DAB precipitate filled the perinuclear
cytoplasm, the dendrites and the dendritic spines of the principal cells
in all areas. DAB reaction product was not present in mitochondria,
but could often be seen enriched around smooth endoplasmic
reticulum cisternae (Fig. 4A). In the hippocampus we never found
FAAH-immunoreactive axon terminals. Immunogold staining, which
allows high spatial resolution, localized FAAH primarily on the
cytoplasmic surface of smooth endoplasmic reticulum cisternae and on
the cytoplasmic surface of mitochondrial outer membranes. Gold
signal could be detected only sparsely on the cytoplasmic side of the
cell surface membranes. A quantitative analysis of the distribution of
gold particles among different compartments, shown in Table 1,
validates these conclusions. We did not observe labelling of glial
processes either in the hippocampus or in the other examined brain
regions at the electron microscopical level.
Fig. 4. Ultrastructural localization of FAAH in the hippocampus. FAAH immunoreactivity (diffuse DAB precipitate) is present in pyramidal cell secondary
dendritic shafts (ds) and spines (sp) in CA3 str. oriens. While almost all shafts and spines are positive for FAAH in this figure, none of the axon terminals showed any
immunoreactivity throughout the examined material. (B–D) Immunogold particles are primarily associated with parts of the endoplasmic reticulum (small arrows)
and with the outer surface of the outer membranes of mitochondria (arrowheads). Labelling was less frequently detected in association with the plasma membrane.
Scale bars, 1 lm (A); 0.5 lm (B and C); 0.2 lm (D).
446 A. I. Gulyas et al.

Light and electron microscopical distribution of MGL
in the hippocampus
A brief description of the findings at the light microscopical level was
included in an earlier study (Dinh et al., 2002). Here we provide a
more comprehensive description of these results complemented with
electron microscopy.
A light microscopy examination of the immunostaining pattern for
MGL (Fig. 5) suggests that this enzyme is associated with presynaptic
axon terminals: principal cell bodies are negative for MGL. In the
CA1 and CA3 areas the MGL-negative primary dendrites of the
pyramidal cells can even be followed into the dendritic layers
expressing a punctate neuropil staining. A dense, punctate, axon
terminal-associated neuropil labelling is present in all other hippo-
campal layers, except the molecular layer of the dentate gyrus and the
str. lacunosum-moleculare of the CA3 and CA1 areas. The dense
MGL staining terminates abruptly at the CA1 ⁄ subiculum border
(Fig. 5D, broken line), suggesting that the signal derives from the
presence of MGL in the axon terminals of CA3 pyramidal cells
forming recurrent collaterals in the CA3 area and in the hilus as well as
giving rise to the Schaffer collaterals in the CA1 subfield. The
presence of large, MGL-positive varicosities in the CA3 str. lucidum
(Fig. 5B and E, double arrowheads) suggests that axon terminals of
granule cells, i.e. the mossy fibers, also express MGL.
Besides the dense punctate neuropil labelling, a more distinct,
stronger signal is present in a subset of axon terminals in all
hippocampal layers. Intensively stained varicosities can be found
primarily around principal cell somata and resemble inhibitory cell
terminals forming pericellular baskets (arrowheads on Fig. 5A–C).
Pericellular baskets are also present around some of the hilar neurons
(Fig. 5A, white arrowheads) that are otherwise negative for MGL.
Labelled boutons can also be identified in association with thick,
unstained principal cell primary dendrites in the CA1 and CA3 areas
(Fig. 5C, double arrowheads). Occasionally, neuronal somata with
features of interneurons are visualized by the staining (Fig. 5C). In
these neurons the signal fills the endoplasmic reticulum in the cell
body and proximal dendrites.
Our prediction from light microscopy, i.e. that MGL is present
primarily in principal cell axon terminals, was unequivocally demon-
strated at the electron microscopical level. Figure 6 shows that, in the
CA1 area, axon terminals forming asymmetrical synapses with heads
of pyramidal cell spines contain MGL. The DAB reaction end-product
homogeneously filled vesicle-containing axon terminals, without any
evident compartmental restriction. MGL-positive axon terminals
forming asymmetrical synapses could also be found on inhibitory
cell dendrites in all hippocampal layers. The presence of MGL in the
axons of dentate granule cells has also been documented by electron
microscopy. As shown in Fig. 7C, mossy fibres that contact the thorny
excrescences of CA3 pyramidal cells in str. lucidum were densely
filled with DAB reaction end-product. As in the case of FAAH we
could find no MGL labelling in glial processes in none of the
examined brain areas.
The light microscopical finding that the neuropil staining for MGL
stopped abruptly at the CA1 ⁄ subiculum border suggested that
Schaffer collaterals, but not CA1 pyramidal cells axons, contain this
enzyme. We further tested this possibility by a careful electron
microscopic examination of asymmetrical synaptic inputs to horizon-
tal interneuron dendrites in CA1 str. oriens. A subset of these
interneurons was shown earlier to receive glutamatergic inputs mostly
if not exclusively from local CA1 pyramidal cells (Blasco-Ibanez &
Freund, 1995). Indeed, we found several dendrites of this type, which
received asymmetrical synapses largely from MGL-negative boutons
(Fig. 6D). Thus, CA1 pyramidal cell axons appear to lack MGL
immunoreactivity.
The presence of MGL in subsets of inhibitory axon terminals,
identified as the stained puncta in all layers of the CA1 area at the
light microscopical level, has also been confirmed. Figure 7A and B
demonstrates that MGL-positive terminals can be found both on
somata (Fig. 7A) and axon initial segments (Fig. 7B) of principal
cells. However, as shown in Fig. 7A, not all perisomatic inhibitory
terminals are MGL-positive. Two subpopulations of perisomatically
terminating inhibitory cell types have been described so far,
containing either PV or CCK in their terminals (Acsady et al.,
1996). CB
1
receptors were shown to be present only in the CCK-
containing subset (Katona et al., 1999). In order to check whether
the presence of MGL in perisomatic terminals correlates with the
presence of these markers, we carried out double-immunostaining
for both MGL and CCK. As shown in Fig. 8, in the case of
perisomatic terminals the pre-embedding immunogold particles
indicating the presence of CCK could always be found in terminals
immunoreactive for MGL (labelled with diffuse DAB precipitation)
in the CA3–CA1 areas. Thus, MGL is present in the CCK ⁄ CB
1
-
immunoreactive basket cell terminals. However, some PV-containing
boutons are also likely to be immunoreactive for MGL, as CCK-
negative ⁄ MGL-positive boutons were also found on the somata. In
addition, chandelier cell axons innervating the axon initial segments
of principal cells are known to contain PV, and they were also
immunoreactive for MGL (Fig. 7B). It is important to note that,
besides principal cells, inhibitory cell somata and dendrites were also
innervated by MGL-containing inhibitory axons. Both MGL-negative
and MGL-positive boutons forming symmetrical synapses have been
found in the dendritic layers (Fig. 6B and C).
Distribution of FAAH and MGL in the amygdala
We found a significant difference in the intensity of FAAH-
immunoreactivity between the basolateral and the central amygdala.
While in the basolateral amygdala a strong cellular and neuropil
staining was present outlining the structure, in the central amygdala
the signal was very weak, represented by occasional neurons
showing faint signal (Fig. 9). The texture of the labelling in the
basolateral amygdala was similar to the hippocampus and cerebel-
lum: the cytoplasm and the proximal dendrites, as well as the
neuropil showed granular ⁄ reticular staining. It is important to note
here that the regional distribution of FAAH matches the distribution
of CB
1
receptors (see inset in Fig. 9A, photographed from the
same material as Katona et al., 2001) in the examined amygdala
nuclei.
Similarly to FAAH, the distribution of MGL also matched the
presence or absence of CB
1
receptors in the basolateral and central
amygdala (compare Figs 9 and 10). A punctate MGL staining was
present in the basolateral amygdala, but was lacking in the central
amygdala. Other amygdala nuclei showed only a background
Table 1. Distribution of FAAH immunogold labelling in the hippocampus
Surface
membrane
Smooth
endoplasmic
reticulum
Mitochondrial
membrane Cytoplasm Total
Gold particles (n) 98 447 142 213 900
Distribution (%) 10.9 49.8 15.8 23.7 100
FAAH, fatty acid amide hydrolase.
Compartmentalization of endocannabinoid hydrolysis 447

level signal. Punctate staining was present in the neuropil and
often surrounded somata. No signal could be seen in neuron
cytoplasm.
The localization of the two enzymes at the ultrastructural level was
studied in the basolateral amgdala using DAB as a chromogen for the
immunoperoxidase reaction (Fig. 10C–F). Similarly to the hippocam-
Fig. 5. Distribution of MGL in the hippocampus. The presence of a punctate signal in the neuropil and positive pericellular basket terminals characterizes MGL
immunostaining. Principal cell bodies and dendrites remained negative indicating that MGL is likely located in axon terminals. (A) In the dentate gyrus (DG)
pericellular baskets surround granule cells (black arrowheads) and some hilar neurons (white arrowheads). Note that some hilar neurons (asterisks) are not
surrounded by MGL-positive terminals. On these cells the proximal dendrites (white arrows) are also devoid of MGL-positive terminals. (B) In the CA3 area, in
addition to the neuropil labelling in str. radiatum and oriens, MGL-containing, pericellular baskets surround the pyramidal cells. In str lucidum, MGL staining
visualizes the mossy terminals (double arrowheads). (C) In the CA1 subfield the neuropil labelling is similar to CA3. Here, pericellular baskets (arrowheads) in str.
pyramidale and immunoreactive individual axon terminals in str. radiatum (double arrowheads) are also visible. The thick apical dendrites of pyramidal cells (white
arrows) are negative for MGL. (D) The micrograph shows that MGL signal disappears at the CA1 area ⁄ subiculum border, i.e. where Schaffer collaterals end,
suggesting that the neuropil staining in CA1–3 derives mostly from Schaffer collaterals. The axons of CA1 pyramidal cells projecting to the subiculum appear to lack
MGL immunoreactivity. In str. lacunosum-moleculare and in the molecular layer of the dentate gyrus the signal is much weaker for MGL, suggesting that entorhinal
afferents also lack this enzyme. (E) In the subiculum the neuropil labelling is considerably less dense than in CA1 str. radiatum. Most probably only axon terminals
of inhibitory neurons are stained. They often surround unstained somata (arrowheads). Scale bars, 50 lm (A–C and E); 500 lm (D); Abbreviations: hil., hilus; s.g.,
str. granulosum; s.l., str. lucidum; s.o., str. oriens; s.p., str. pyramidale; s.r., str. radiatum; subic., subiculum.
448 A. I. Gulyas et al.

Compartmentalization of endocannabinoid hydrolysis 449
Fig. 6. Exclusive presynaptic localization of MGL in the hippocampus. (A) The spines (sp) of a second order pyramidal cell dendrite (Pd) in the CA1 region
receive asymmetrical synapses from MGL-immunoreactive axon terminals (arrowheads). (B and C) Axon terminals forming symmetrical synapses (asterisks) on
pyramidal cell (Pd in C) or interneuron (Id in B) dendrites are often negative for MGL, but positive examples are also common. Arrowheads label the positive
terminals establishing asymmetrical contacts on
spines. (D) The horizontally orientated inhibitory cell dendrite (Id) in str. oriens of the CA1 subfield is densely
covered with MGL-negative axon terminals (asterisks) forming asymmetrical synapses. These boutons likely originate from local CA1 pyramidal cell collaterals that
are known to account for the majority of synaptic input of these interneurons. A neighbouring dendritic spine is innervated by an MGL-positive axon terminal,
probably of Schaffer collateral origin. Scale bars, 0.5 lm.
pus, the distribution of the signal for the two proteins was
complementary. FAAH was present in dendrites of different diameter
and in somata. The DAB precipitate often accumulated in the vicinity
of mitochondria (Fig. 10C and D). In contrast, MGL signal was seen
in axon terminals loaded with vesicles and forming either symmetrical
or asymmetrical synapses on MGL-negative dendritic profiles. This
suggests that MGL is present in subsets of both excitatory and
inhibitory terminals (Fig. 10E and F).
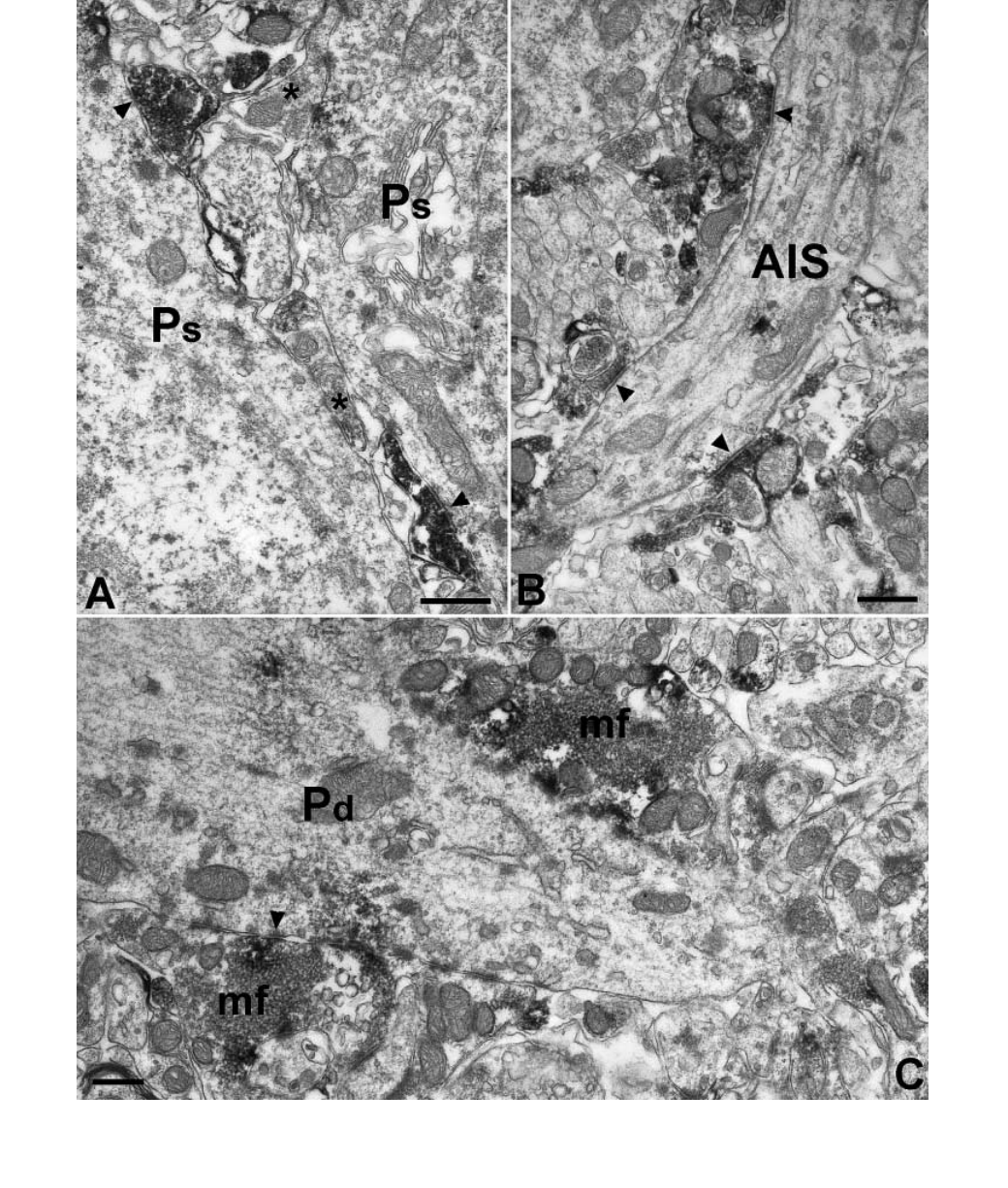
Fig. 7. The majority of perisomatic inhibitory axon terminals and the mossy terminals are immunoreactive for MGL. (A) MGL-positive basket cell terminals
form symmetrical synapses (arrowheads) on the somata of pyramidal cells (Ps) in the CA1 area. Note that MGL-negative (asterisks) basket cell terminals are also
present. (B) Axon terminals of chandelier cells forming symmetrical synapses (arrowheads) on pyramidal cell axon initial segments (AIS) are also positive for
MGL. (C) Two mossy fibre terminals (mf) surround the thick proximal dendrite (Pd) of a pyramidal cell in CA3 str. lucidum. Scale bars, 0.5 lm.
450 A. I. Gulyas et al.

Fig. 8. MGL is present in CCK-containing basket cell terminals in the CA1–3 regions. MGL was visualized using immunoperoxidase reaction and DAB as a
chromogen producing a diffuse electron-dense end-product, whereas CCK immunoreactivity is represented by silver-intensified immunogold particles (arrows). A
large proportion of the MGL-positive boutons synapsing on pyramidal cell bodies (Pc) were also immunoreactive for CCK, but additional basket cell terminals
(asterisks) negative for both antigens were also visible. These terminals most probably derive from PV-IR basket cells. Scale bars, 0.5 lm.
Compartmentalization of endocannabinoid hydrolysis 451

Fig. 9. Fatty acid amide hydrolase (FAAH) expression correlates with the presence of cannabinoid receptors subtype 1 (CB
1
) receptor immunoreactivity in
divisions of the amygdala. (A) Neurons of the basolateral amygdala (blA) are heavily immunoreactive for FAAH in contrast to the central amygdala (cA), where
hardly any cells contain this enzyme. The inset shows the correlation with the relative distribution of CB
1
receptor in the different nuclei. The boxed areas in A are
shown at higher magnification in B and C. (B) Occasional neuronal staining (arrow) and the lack of neuropil immunoreactivity has been observed in the central
amygdala. (C) Intensively labelled cell bodies (arrows) and primary dendrites are visualized by FAAH immunoreactivity in addition to the immunostained neuropil
in the basolateral amygdala. The same region expresses strong CB
1
receptor immunoreactivity, although this staining is known to be confined to axons (see Katona
et al., 2001). Scale bars, 250 lm (A); 50 lm (B and C).
452 A. I. Gulyas et al.

Compartmentalization of endocannabinoid hydrolysis 453
Fig. 10. Ultrastructural segregation of MGL and FAAH in the amygdala. (A) While there is a dense neuropil signal in the basolateral amygdala (blA), the central
amygdala (cA) shows weak immunoreactivity. Nearby structures express low levels of MGL [bed nucleus of the stria terminalis-intra-amygdaloid division (BSTIA)]
or no MGL at all [medial amygdala nuclei (mA)]. (B) Enlarged view of the boxed area in A. The neuropil staining derives from immunoreactive puncta, possibly
axon terminals some of which form pericellular baskets (arrows) or are present in the neuropil (arrowheads). (C and D) FAAH immunostaining is present
postsynaptically in the amygdala, in dendritic shafts (ds) and spines (sp.). (C) Arrows indicate that in larger dendrites the DAB end-product is accumulated around
mitochondria. Asterisks label FAAH-negative axon terminals. One of them forms an asymmetrical synapse (arrowhead) on a labelled spine head. (E and F) MGL
immunostaining is present presynaptically in subsets of axon terminals (white asterisks). (E) A putative inhibitory terminal, as it forms a symmetrical synapse
(double arrowhead) on a cell body. The left terminal on (F) forms an asymmetrical synapse on a dendritic spine, identifying it as an excitatory terminal. Scale bars,
500 lm (A); 10 lm (B); 0.5 lm (C and D); 0.2 lm (E and F).
Light and electron microscopic distribution of FAAH
in the cerebellum
In contrast to the finding of Egertova et al. (2003), we found that
not only the somata, but the entire dendritic arbor of Purkinje cells
expressed high levels of FAAH immunoreactivity, resulting in a
layer-selective labelling in the cerebellum (Fig. 11). Similar to the
hippocampus, the immunostaining showed a granular-reticular
pattern that was especially evident in the cytoplasm of the large
Purkinje cells. In the granule cell layer occasional axon terminals
proved to be FAAH-positive, primarily at the border of the Purkinje
cell ⁄ granule cell layers. These axon terminals were large and often
surrounded FAAH-negative, small, horizontally elongated cell
bodies in the Purkinje cell layer (inset in Fig. 11). Granule cells
showed faint signal, close to the threshold of immunocytochemical
detection. We also examined the immunostaining of different
interneuron types located in various cerebellar layers, i.e. Golgi,
basket and stellate cells (Fig. 11C and D). Here the staining
intensity was similar to the signal in the granule cells; thus,
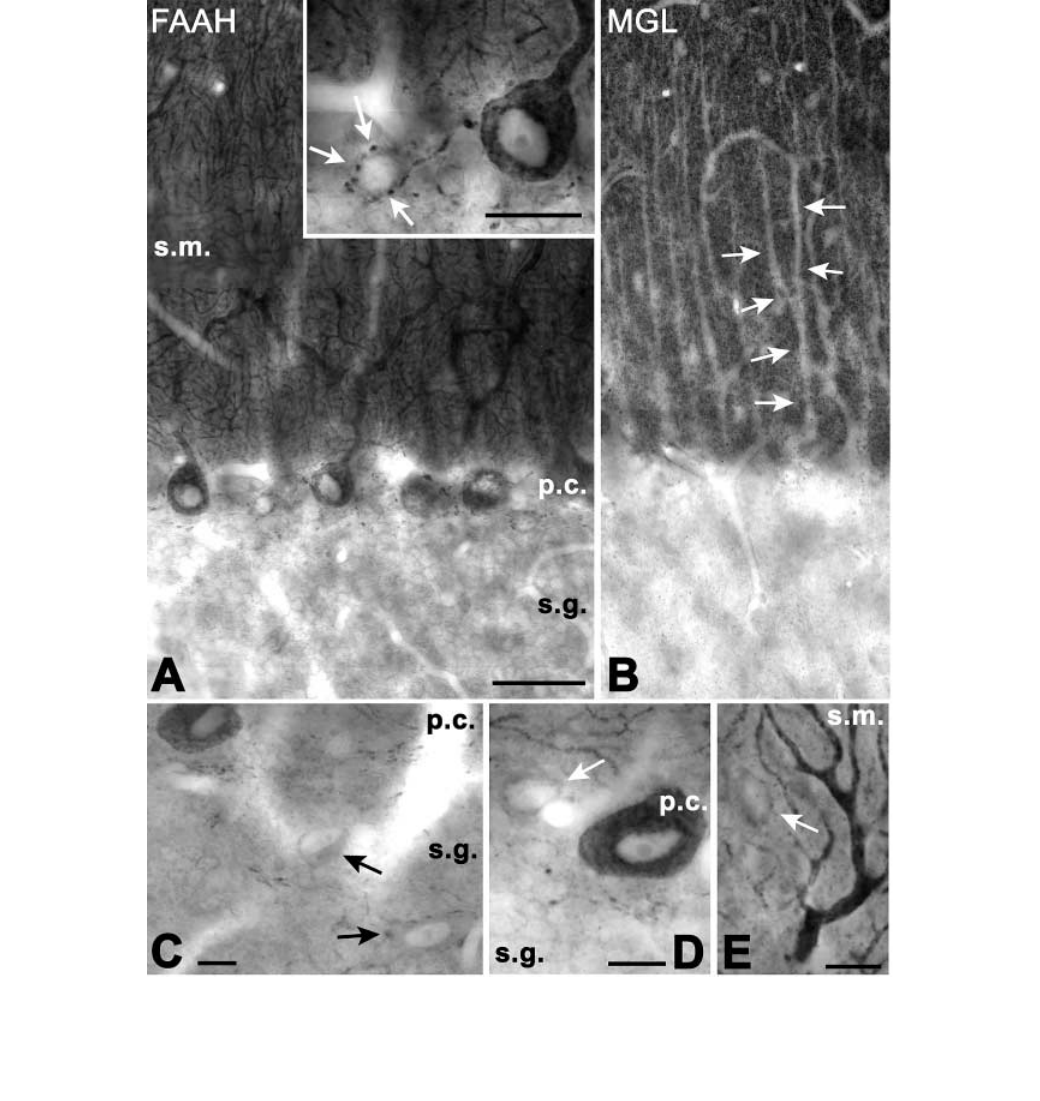
Fig. 11. Complementary distribution of fatty acid amide hydrolase (FAAH) and monoglyceride lipase (MGL) in str. moleculare of the cerebellum. (A) Purkinje cell
bodies and their entire dendritic arbor is strongly immunostained for FAAH in the molecular (s.m.) and Purkinje cell (p.c.) layers, in contrast to the occasional axonal
labelling (arrows in inset) surrounding small FAAH-negative cell bodies just below the Purkinje cell layer. (B) The MGL-negative Purkinje cell dendrites (arrows)
stand out from the darkly immunoreactive neuropil that likely consists of MGL-positive axon terminals. (C–E) Interneurons (arrows) in the granule
(C) and Purkinje cell layer (D), as well as in the molecular layer (E) showed weak if any immunoreactivity, close to the immunocytochemical detection threshold.
Scale bars, 40 lm (A); 20 lm (B); 10 lm (C–E). Abbreviations: p.c., Purkinje cell layer; s.g., str. granulosum; s.m., str. moleculare.
454 A. I. Gulyas et al.

Compartmentalization of endocannabinoid hydrolysis 455
Fig. 12. Ultrastructural localization of FAAH in the cerebellum. Immunoperoxidase reaction using DAB as a chromogen visualized Purkinje cell dendrites (ds) and
spines (large arrows) in the molecular layer (A and B) and a few axon terminals (C) at the border of the Purkinje and granule cell layers. The presence of dendritic
spines (large arrows) identifies the branching dendrite (outlined by
small arrows) as belonging to a Purkinje cell in A. (C) A FAAH-positive axon terminal (at)
forms a synapse (white arrowheads) on a FAAH-negative dendrite (ds) in stratum granulosum. (D and E) Immunogold particles indicate that FAAH is associated
with membranes, as expected from a protein with transmembrane domains. It is primarily located on the outer surface of the outer membranes of mitochondria
(arrowheads) and on the surface of the endoplasmic reticulum (small arrows). The plasma membrane and the stacked cisternae of the smooth endoplasmic reticulum
(large arrows) rarely showed any labelling for FAAH. Scale bars, 1 lm (A, D and E); 0.5 lm (B, C).

compared to the Purkinje cells, the inhibitory interneurons can be
considered FAAH-negative.
The electron microscopic examination confirmed our light micro-
scopic finding that in the molecular and Purkinje cell layers only the
dendrites of the Purkinje cells express FAAH (Fig. 12A, B, D and E).
Immunoreactivity was present in the thick primary and the thinner
higher order dendrites of the Purkinje cells. The signal in the spines
was not as evident as in hippocampal pyramidal cells. The presence of
FAAH in the granule cell layer axons was also confirmed at the
electron microscopic level (Fig. 12C). The labelled axon terminals
formed synapses on FAAH-negative somata and dendrites.
The immunogold signal, similar to the hippocampus, was located
primarily on the cytoplasmic surface of the mitochondrial outer
membranes and on the cytoplasmic surface of smooth endoplasmic
reticulum cisternae. The surface membranes and the stacked cisternae
of the smooth endoplasmic reticulum were mostly devoid of FAAH
(Fig. 12D and E). The quantitative results on the distribution of the
gold particles, shown in Table 2, demonstrate that the overall picture
is similar to the hippocampus. The difference observed arose from
the higher incidence of gold particles over mitochondrial membranes
at the expense of labelling over the surface membrane and the
cytoplasm.
Light microscopical distribution of MGL in the cerebellum
Similarly to the hippocampus, the localization of MGL and FAAH is
also complementary in the cerebellum. This can be clearly seen in
Fig. 11. In Fig. 11B, the negative Purkinje cell dendrites stand out
from a background of MGL-positive puncta in str. moleculare, making
it possible to follow even the second order thinner dendrites of the
cells. A considerably less dense punctate staining was present in str.
granulosum. Clusters of immunolabelled puncta outlined small, MGL-
negative patches that correspond to glomeruli. Thus, these puncta are
likely to be Golgi cell terminals.
Discussion
The major finding of our study is that FAAH is localized in the soma-
dendritic compartment, whereas MGL is localized in axons. Further-
more, our results also allow various conclusions to be made. First, in
the hippocampus, FAAH is present postsynaptically in somata and
dendrites of principal neurons, but is absent from inhibitory interneu-
rons. Second, in the cerebellum, Purkinje cell somata and dendrites, as
well as a small subpopulation of axon terminals express FAAH. Third,
in the hippocampus and cerebellum FAAH is located primarily on the
cytosolic surface of smooth endoplasmic reticulum cisternae and
mitochondrial outer membranes, with only a very small proportion of
the enzyme associated with cell membranes. Fourth, the distribution of
MGL parallels that of CB
1
receptors and FAAH at the regional level,
whereas it is complementary to FAAH distribution at the ultrastruc-
tural level. Fifth, in the hippocampus MGL is present in axon
terminals of granule cells and CA3 pyramidal cells throughout their
entire axonal arborization. A subpopulation of inhibitory axon
terminals, including those of CCK-immunoreactive basket cells and
axo-axonic cells, also express MGL. Sixth, in the cerebellum MGL
immunostaining is present only in str. moleculare in the form of
punctate axonal labelling. Seventh, in the amygdala FAAH and MGL
expression matches that of the CB
1
receptors, i.e. they are present in
the basolateral nucleus, but are nearly absent from the central nucleus.
Eighth, the ultrastructural distribution of the two materials in the
amygdala, similar to the hippocampus, is complementary: i.e. FAAH
is located postsynaptically, MGL presynaptically.
FAAH is associated with intracellular membranes of principal
neurons
We confirmed earlier results (Egertova et al., 1998; Tsou et al., 1998b)
suggesting that the distributions of CB
1
and FAAH, while overlapping
in several regions of the brain, are complementary at the cellular level.
In the hippocampus and cerebellum, CB1 receptors are located
presynaptically on axon terminals of subsets of inhibitory neurons
(Tsou et al., 1998a; Katona et al., 1999) and in the cerebellum
probably also on excitatory axons, whereas FAAH is found in
dendrites of postsynaptic principal neurons. As expected from its
structure, FAAH may be located on the cytoplasmic surface of
membranes. However, quite unexpectedly, the enzyme was primarily
associated with intracellular membranes, including smooth endoplas-
mic reticulum cisternae and the external surface of the mitochondria,
but not with the plasma membrane.
X-ray crystallography studies suggest that anandamide may access
the active site of FAAH from the lipid membrane (Bracey et al., 2002).
During the breakdown phase of endocannabinoids fatty acid amides can
be internalized by endocytosis into lipid vesicles that later fuse with
endoplasmic reticulum and mitochondria membranes, where FAAH
would degrade these lipids. Alternatively, anandamide must cross the
cell membrane and travel through the cytosol to the endoplasmic
reticulum. The first step may involve a transmembrane transport system
(Beltramo et al., 1997; Hillard et al., 1997), which remains to be
molecularly characterized, while the second might implicate one or
more intracellular lipid-binding protein(s). It is important to point out
that, in addition to anandamide, FAAH is responsible for the hydrolysis
of other fatty acid amides with potent biological actions. These include
the endogenous ligand for peroxisome-proliferator-activated receptors,
oleoylethanolamide (Rodriguez de Fonseca et al., 2001; Fu et al.,
2003), and the anti-inflammatory ⁄ analgesic mediator palmitoyletha-
nolamide (Mazzari et al., 1996; Calignano et al., 2000). Although 2-AG
is hydrolysed by FAAH in broken cell preparations (Goparaju et al.,
1998), FAAH – ⁄ – mice have normal brain 2-AG levels (Lichtman et al.,
2002), indicating that FAAH does not contribute to 2-AG degradation
in vivo. Our results, showing that FAAH and MGL are preferentially
localized in distinct neuronal compartments, provide a possible
explanation for this discrepancy.
Table 2. Distribution of FAAH immunogold labelling in the cerebellum
Surface
membrane
Smooth
endoplasmic
reticulum
Stacked lamellae
of endoplasmic
reticulum
Mitochondrial
membrane Cytoplasm Total
Gold particles (n) 9 265 11 210 67 562
Distribution (%) 1.6 47.2 2.0 37.4 11.9 100
FAAH, fatty acid amide hydrolase.
456 A. I. Gulyas et al.
The lack of FAAH in GABAergic interneurons is going to provide
good guidance in the identification of the precise function(s) of FAAH.
For example, the presence or lack of depolarization-induced suppres-
sion of inhibition, and the associated endocannabinoid release, may
shed light on the involvement of FAAH in this phenomenon.
MGL is located presynaptically in axons of subsets
of excitatory and inhibitory neurons
The distribution of MGL immunoreactivity in the hippocampus and
amygdala suggests that this endocannabinoid-degrading enzyme has
a selective presynaptic localization. It can be found in the axons of
dentate granule cells and CA3 pyramidal cells, i.e. in the mossy
fibre terminals and in the Schaffer collaterals. MGL is present
presynaptically in the cerebellum as well as in the molecular layer
where parallel and climbing fibres terminate. In the hippocampus,
cannabimimetic agents reduce glutamatergic EPSCs in CB
1
– ⁄ –
mice (Hajos et al., 2001), suggesting that glutamatergic axon
terminals express a novel cannabinoid-sensitive receptor. In the
cerebellum, depolarization-induced suppression of excitation has
been demonstrated on parallel and climbing fibres terminating on
Purkinje cells (Kreitzer & Regehr, 2001b), but similar effects in the
hippocampus could only be evoked in a single study using very
long depolarizing pulses (Ohno-Shosaku et al., 2002). The effect
could be modulated by cannabinoid antagonists and agonists, and
was absent in CB
1
KO mice. Thus, the release of glutamate from
excitatory terminals might be under the control of endocannabinoids
in both regions. The presence of MGL in glutamatergic fibres might
be necessary to terminate the effect of endocannabinoids on
excitatory transmission. The fact that MGL is not present in str.
lacunosum-moleculare, neither in the dentate molecular layer nor in
the subiculum, suggests that inputs from the entorhinal cortex and
from the CA1 area are not controlled by endocannabinoids. This
prediction has yet to be tested, as no data are available on
the existence of depolarization-induced suppression of excitation or
the modulation of glutamate release in the perforant pathway or the
subiculum.
Besides excitatory terminals, subsets of hippocampal and amygdala
interneurons express MGL in their axon terminals. Two populations of
axon terminals proved to contain MGL. The presence of MGL in the
CCK-positive subpopulation of interneurons can be explained by the
fact that these cells also express CB
1
receptors (Katona et al., 1999).
Thus, similarly to glutamatergic terminals, MGL might terminate the
effect of endocannabinoids in this population of axon terminals. The
presence of MGL in the axo-axonic cell terminals needs an alternative
explanation, as these inhibitory axons do not express CB
1
receptors. In
the granule cell layer of the cerebellum we observed a punctate
terminal labelling surrounding negative areas with the size of
glomeruli formed by granule cell dendrites, mossy fibres and Golgi
cell axon terminals. Because the inhibitory terminals of Golgi cells are
located on the periphery of the glomeruli (Hamori & Takacs, 1989),
the observed punctate signal among the granule cells most probably
derives from the Golgi cell terminals and preterminal axons. Thus, it
seems that at least one subpopulation of cerebellar inhibitory cells
expresses FAAH in their axons.
Because the ultrastructural localization of synthesis, mechanism of
release, site and speed of transport of endocannabinoids are not yet
known, the functional implications of these findings are limited at
present. We can only speculate as to why the two enzymes of
endocannabinoid hydrolysis are located in complementary compart-
ments. Based on available evidence, we suggest that FAAH may set the
resting level of anandamide close to its sites of synthesis, while MGL
may help to inactivate 2-AG close to its sites of action. This hypothesis
is in agreement with the cellular localization of FAAH in proximity of
Ca
2+
stores (mitochondria, endoplasmic reticulum), where Ca
2+
-
dependent anandamide synthesis might take place. However, to fully
understand the cycle of endocannabinoids synthesis, release and
deactivation, it will be necessary to characterize all components of this
cycle, including synthetic enzymes and transporters, and provide a
detailed description of their localization and kinetics of actions.
Acknowledgements
We are grateful to Dr I. Katona for valuable contributions at preliminary stages
of the study, to Dr N. Ha´jos for helpful discussions, and to Mrs K. Lengyel,
Ms E. Simon, Ms K. Ivanyi and Mr Gy. Goda for the excellent technical
assistance. This work was supported by the Howard Hughes Medical Institute
(USA), NIH (MH 54 671, NS30549, DA13173, DA15197, DA-12493 and
DA-12447), Philip Morris External Research Program and OTKA (T034638
and T032251, Hungary). F.B. was supported by the University of Naples
‘Federico II’, School of Medicine PhD program.
Abbreviations
2-AG, sn-2-arachidonoyl-glycerol; CB
1
, cannabinoid receptors subtype 1;
CCK, cholecystokinin; DAB, diaminobenzidine; FAAH, fatty acid amide
hydrolase; GABA, c-aminobutyric acid; MGL, monoglyceride lipase; PB,
phosphate buffer; PV, parvalbumin.
References
Acsady, L., Gorcs, T.J. & Freund, T.F. (1996) Different populations of
vasoactive intestinal polypeptide-immunoreactive interneurons are specia-
lized to control pyramidal cells or interneurons in the hippocampus.
Neuroscience, 73, 317–334.
Beltramo, M., Stella, N., Calignano, A., Lin, S.Y., Makriyannis, A. & Piomelli,
D. (1997) Functional role of high-affinity anandamide transport, as revealed
by selective inhibition. Science, 277, 1094–1097.
Blasco-Ibanez, J.M. & Freund, T.F. (1995) Synaptic input of horizontal
interneurons in stratum oriens of the hippocampal CA1 subfield: structural
basis of feed-back activation. Eur. J. Neurosci., 7, 2170–2180.
Bracey, M.H., Hanson, M.A., Masuda, K.R., Stevens, R.C. & Cravatt, B.F.
(2002) Structural adaptations in a membrane enzyme that terminates
endocannabinoid signaling. Science, 298, 1793–1796.
Cadas, H., di Tomaso, E. & Piomelli, D. (1997) Occurrence and biosynthesis of
endogenous cannabinoid precursor, N-arachidonoyl phosphatidylethanola-
mine, in rat brain. J. Neurosci., 17, 1226–1242.
Calignano, A., La Rana, G., Loubet-Lescoulie, P. & Piomelli, D. (2000) A role
for the endogenous cannabinoid system in the peripheral control of pain
initiation. Prog. Brain Res., 129, 471–482.
Cravatt, B.F., Demarest, K., Patricelli, M.P., Bracey, M.H., Giang, D.K.,
Martin, B.R. & Lichtman, A.H. (2001) Supersensitivity to anandamide and
enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide
hydrolase. Proc. Natl Acad. Sci. USA, 98, 9371–9376.
Devane, W.A., Dysarz, F.A. 3rd, Johnson, M.R., Melvin, L.S. & Howlett, A.C.
(1988) Determination and characterization of a cannabinoid receptor in rat
brain. Mol. Pharmacol., 34, 605–613.
Devane,W.A.,Hanus,L.,Breuer,A.,Pertwee,R.G.,Stevenson,L.A.,Griffin,G.,
Gibson, D., Mandelbaum, A., Etinger, A. & Mechoulam, R. (1992) Isolation
and structure of a brain constituent that binds to the cannabinoid receptor.
Science, 258, 1946–1949.
Dinh, T.P., Carpenter, D., Leslie, F.M., Freund, T.F., Katona, I., Sensi, S.L.,
Kathuria, S. & Piomelli, D. (2002) Brain monoglyceride lipase participating
in endocannabinoid inactivation. Proc. Natl Acad. Sci. USA, 99, 10819–
10824.
Egertova, M., Cravatt, B.F. & Elphick, M.R. (2003) Comparative analysis of
fatty acid amide hydrolase and cb(1) cannabinoid receptor expression in
the mouse brain: evidence of a widespread role for fatty acid amide
hydrolase in regulation of endocannabinoid signaling. Neuroscience, 119,
481–496.
Compartmentalization of endocannabinoid hydrolysis 457
Egertova, M., Giang, D.K., Cravatt, B.F. & Elphick, M.R. (1998) A new
perspective on cannabinoid signalling: complementary localization of fatty
acid amide hydrolase and the CB1 receptor in rat brain. Proc. R. Soc. Lond. B
Biol. Sci., 265, 2081–2085.
Freund, T.F. & Buzsaki, G. (1996) Interneurons of the hippocampus.
Hippocampus, 6, 345–470.
Fu, J., Gaetani, S., Oveisi, F., Lo Verme, J., Serrano, A., Rodriguez de Fonseca, F .,
Rosengarth, A., Luecke, H., Di Giacomo, B., Tarzia, G. & Piomelli, D.
(2003) Oleylethanolamide regulates feeding and body weight through
activation of the nuclear receptor PPAR-alpha. Nature, 425, 90–93.
Goparaju, S.K., Ueda, N., Yamaguchi, H. & Yamamoto, S. (1998) Anandamide
amidohydrolase reacting with 2-arachidonoylglycerol, another cannabinoid
receptor ligand. FEBS Lett., 422, 69–73.
Hajos, N., Katona, I., Naiem, S.S., MacKie, K., Ledent, C., Mody , I. & Freund, T .F.
(2000) Cannabinoids inhibit hippocampal GABAergic transmission and
network oscillations. Eur. J. Neurosci., 12, 3239–3249.
Hajos, N., Ledent, C. & Freund, T.F. (2001) Novel cannabinoid-sensitive
receptor mediates inhibition of glutamatergic synaptic transmission in the
hippocampus. Neuroscience, 106, 1–4.
Hamori, J. & Takacs, J. (1989) Two types of GABA-containing axon terminals
in cerebellar glomeruli of cat: an immunogold-EM study. Exp. Brain Res.,
74, 471–479.
Herkenham, M., Lynn, A.B., Little, M.D., Johnson, M.R., Melvin, L.S.,
de Costa, B.R. & Rice, K.C. (1990) Cannabinoid receptor localization in
brain. Proc. Natl Acad. Sci. USA, 87, 1932–1936.
Hillard, C.J., Edgemond, W.S., Jarrahian, A. & Campbell, W.B. (1997)
Accumulation of N-arachidonoylethanolamine (anandamide) into cerebellar
granule cells occurs via facilitated diffusion. J. Neurochem., 69, 631–638.
Hoffman, A.F. & Lupica, C.R. (2000) Mechanisms of cannabinoid inhibition of
GABA(A) synaptic transmission in the hippocampus. J. Neurosci., 20,
2470–2479.
Katona, I., Rancz, E.A., Acsady, L., Ledent, C., Mackie, K., Hajos, N. &
Freund, T.F. (2001) Distribution of CB1 cannabinoid receptors in the
amygdala and their role in the control of GABAergic transmission.
J. Neurosci., 21, 9506–9518.
Katona, I., Sperlagh, B., Sik, A., Kafalvi, A., Vizi, E.S., Mackie, K. & Freund,
T.F. (1999) Presynaptically located CB1 cannabinoid receptors regulate
GABA release from axon terminals of specific hippocampal interneurons.
J. Neurosci., 19, 4544–4558.
Kreitzer, A.C. & Regehr, W.G. (2001a) Cerebellar depolarization-induced
suppression of inhibition is mediated by endogenous cannabinoids.
J. Neurosci., 21, RC174.
Kreitzer, A.C. & Regehr, W.G. (2001b) Retrograde inhibition of presynaptic
calcium influx by endogenous cannabinoids at excitatory synapses onto
Purkinje cells. Neuron, 29, 717–727.
Lichtman, A.H., Hawkins, E.G., Griffin, G. & Cravatt, B.F. (2002)
Pharmacological activity of fatty acid amides is regulated, but not mediated,
by fatty acid amide hydrolase in vivo. J. Pharmacol. Exp. Ther., 302, 73–79.
Mailleux, P. & Vanderhaeghen, J.J. (1992) Distribution of neuronal
cannabinoid receptor in the adult rat brain: a comparative receptor binding
radioautography and in situ hybridization histochemistry. Neuroscience, 48,
655–668.
Matsuda, L.A., Lolait, S.J., Brownstein, M.J., Young, A.C. & Bonner, T.I.
(1990) Structure of a cannabinoid receptor and functional expression of the
cloned cDNA. Nature, 346, 561–564.
Mazzari, S., Canella, R., Petrelli, L., Marcolongo, G. & Leon, A. (1996)
N-(2-hydroxyethyl) hexadecanamide is orally active in reducing edema
formation and inflammatory hyperalgesia by down-modulating mast cell
activation. Eur. J. Pharmacol., 300, 227–236.
Misner, D.L. & Sullivan, J.M. (1999) Mechanism of cannabinoid effects on
long-term potentiation and depression in hippocampal CA1 neurons.
J. Neurosci., 19, 6795–6805.
Ohno-Shosaku, T., Maejima, T. & Kano, M. (2001) Endogenous cannabinoids
mediate retrograde signals from depolarized postsynaptic neurons to
presynaptic terminals. Neuron, 29, 729–738.
Ohno-Shosaku, T., Tsubokawa, H., Mizushima, I., Yoneda, N., Zimmer, A. &
Kano, M. (2002) Presynaptic cannabinoid sensitivity is a major determinant
of depolarization-induced retrograde suppression at hippocampal synapses.
J. Neurosci., 22, 3864–3872.
Patricelli, M.P., Lashuel, H.A., Giang, D.K., Kelly, J.W. & Cravatt, B.F. (1998)
Comparative characterization of a wild type and transmembrane domain-
deleted fatty acid amide hydrolase: identification of the transmembrane
domain as a site for oligomerization. Biochemistry, 37, 15177–15187.
Rodriguez de Fonseca, F., Navarro, M., Gomez, R., Escuredo, L., Nava, F.,
Fu, J., Murillo-Rodriguez, E., Giuffrida, A., LoVerme, J., Gaetani, S.,
Kathuria, S., Gall, C. & Piomelli, D. (2001) An anorexic lipid mediator
regulated by feeding. Nature, 414, 209–212.
Shen, M., Piser, T.M., Seybold, V.S. & Thayer, S.A. (1996) Cannabinoid
receptor agonists inhibit glutamatergic synaptic transmission in rat
hippocampal cultures. J. Neurosci., 16, 4322–4334.
Sloviter, R.S. (1989) Calcium-binding protein (calbindin-D28k) and parvalbu-
min immunocytochemistry: localization in the rat hippocampus with specific
reference to the selective vulnerability of hippocampal neurons to seizure
activity. J. Comp. Neurol., 280, 183–196.
Stella, N. & Piomelli, D. (2001) Receptor-dependent formation of endogenous
cannabinoids in cortical neurons. Eur. J. Pharmacol., 425, 189–196.
Stella, N., Schweitzer, P. & Piomelli, D. (1997) A second endogenous
cannabinoid that modulates long-term potentiation. Nature, 388, 773–778.
Szabat, E., Soinila, S., Happola, O., Linnala, A. & Virtanen, I. (1992) A new
monoclonal antibody against the GABA-protein conjugate shows immunor-
eactivity in sensory neurons of the rat. Neuroscience, 47, 409–420.
Thomas, E.A., Cravatt, B.F., Danielson, P.E., Gilula, N.B. & Sutcliffe, J.G.
(1997) Fatty acid amide hydrolase, the degradative enzyme for anandamide
and oleamide, has selective distribution in neurons within the rat central
nervous system. J. Neurosci. Res., 50, 1047–1052.
Tsou, K., Brown, S., Sanudo-Pena, M.C., Mackie, K. & Walker, J.M. (1998a)
Immunohistochemical distribution of cannabinoid CB1 receptors in the rat
central nervous system. Neuroscience, 83, 393–411.
Tsou, K., Nogueron, M.I., Muthian, S., Sanudo-Pena, M.C., Hillard, C.J.,
Deutsch, D.G. & Walker, J.M. (1998b) Fatty acid amide hydrolase is located
preferentially in large neurons in the rat central nervous system as revealed
by immunohistochemistry. Neurosci. Lett., 254, 137–140.
Wilson, R.I. & Nicoll, R.A. (2001) Endogenous cannabinoids mediate
retrograde signalling at hippocampal synapses. Nature, 410, 588–592.
458 A. I. Gulyas et al.
