
UNDERSTANDING BLOOD ANALYSIS IN DUI AND TRAFFIC
HOMICIDE INVESTIGATIONS
Editor: Patrick Mahaney
Attorney-at-Law, Montgomery, Alabama
Technical Editor: Dr. Jack R. Kalin, PhD, DFTCB
Toxicology Discipline Chief, Alabama Department of Forensic Sciences
Technical Review: Dr. Jimmie L. Valentine, PhD, Medical Pharmacology and Toxicology
Consulting, Ocean Springs, Mississippi
Introduction: Analysis of blood evidence in a DUI, traffic assault, or traffic homicide case is a
critical element of the case for the investigating law enforcement officer as well as the
prosecutor. Blood samples taken from the defendant are a key piece of evidence in establishing
criminal culpability. This document is designed to give the non-scientifically trained law
enforcement officer, prosecutor, or attorney sufficient information to understand the basic
properties of alcohol and blood, a basic understanding of Alabama law regarding legal issues
concerning the admissibility of blood sample evidence, and how blood samples are analyzed.
Understanding Alcohol and Blood: The Basics
Alcohol: Alcohol
1
is one of the oldest substances known to mankind, but its effects are
continually being studied, re-studied, and analyzed. Beverage alcohol is commonly referred to
as “ethanol” or “ethyl alcohol” as well as “alcohol.” Ethanol is one of a family of alcohols
which includes methanol (methyl alcohol or “wood alcohol”), 1-Propanol (propyl alcohol), 1-
utanol (butyl alcohol), 2-Propanol (isopropyl alcohol or “rubbing alcohol”), and ethanediol
(ethylene glycol or “antifreeze”).
1
Origin of the word “alcohol’: The al— in alcohol indicates this is a word of Arabic descent, as is the case with
algebra and alkali; al- being the Arabic definite article corresponding to “the” in English. The origin of —cohol is
less obvious, however. Its Arabic ancestor was kuhl, a fine powder most often made from antimony and used by
women to darken their eyelids; in fact, kuhl has given us the word kohl for such a preparation. Arab chemists came
to use al-kuhl to mean “any fine powder produced in a number of ways, including the process of heating a substance
to a gaseous state and then re-cooling it.”
The English word alcohol, derived through Medieval Latin from Arabic, is first recorded in 1543 in this sense. The
introduction of the word “alcohol” into the English language came from French, and earlier from Medieval Latin,
and is credited to a Latin translation of the works of Rhayzes (865-925), a noted Persian physician, alchemist, and
natural scientist.
The invention of the distillation process to produce ethanol as a beverage is credited to Arab and Persian chemists in
the eighth Century. However, the technique of distillation would not reach Europe until the twelfth century, and its
name from the Arabic “al-kuhl” would become the basis for the later English word “alcohol.” Arabic chemists also
used al-kuhl to refer to other substances such as essences that were obtained by distillation, a sense first found for
English alcohol in 1672. One of these distilled essences, known as “alcohol of wine,” is the constituent of
fermented liquors that causes intoxication. This essence took over the term alcohol for itself, and has come to refer
to the liquor that contains this essence, as well as to a class of chemical compounds such as methanol.
1

Common Alcohol Compounds
Common Name IUPAC Formula
Methyl alcohol Methanol CH
3
OH
Ethyl alcohol Ethanol CH
3
CH
2
OH
n-Propyl alcohol 1-Propanol CH
3
CH
2
CH
2
OH
Isopropyl alcohol 2-Propanol (CH
3
)
2
CHOH
n-Butyl alcohol 1-Butanol CH
3
(CH
2
)
2
CH
2
OH
Ethyl alcohol (ethanol) is a very small molecule that is completely soluble (miscible) in water.
Ethanol is lighter than water. Ethanol has a specific gravity 0.789 while water has a specific
gravity of 1.000. Additionally, ethanol has a boiling point at 78 degrees Celsius as opposed to
water at 100 degrees Celsius. The fact that alcohol is both lighter than water and boils at a lower
boiling point is essential in the distillation process. The main source of consumed alcohol is
commercially prepared beverages: fermented alcoholic beverages and distilled alcoholic
beverages. Beer and wine are typical fermented beverages. In both cases, a natural product
(barley in the case of beer and grapes in the case of wine) is fermented by the addition of yeast
microorganisms. The alcohol that is produced is the waste byproduct of the metabolism of the
yeast’s or bacteria’s consumption of sugars found in the natural product. Throughout the
remainder of this document, the terms ethanol and alcohol may be used interchangeably.
The Fermentation Process:
The understanding of alcohol must begin with the fermentation process. Fermentation of sugars
by yeast is the oldest synthetic organic chemical produced by man. During fermentation, sugar is
converted to drinking alcohol and carbon dioxide is released as gas bubbles. This chemical
change was a great mystery to ancient man because the mixture appeared to be boiling without
heat. It was not until the mid-19
th
Century when the noted French chemist and natural scientist
Louis Pasteur discovered that alcoholic fermentation could occur only in the presence of small
living “ferments” or, as they are known today, yeasts
2
.
2
Yeasts are eukaryotic microorganisms, classified in the kingdom Fungi, with about 1,500 species currently
described; they dominate fungal diversity in the oceans. Most reproduce asexually by budding, although a few do so
by binary fission. Yeasts are unicellular, although some species with yeast forms may become multicellular through
the formation of a string of connected budding cells known as pseudohyphae, or false hyphae as seen in most molds.
Yeast size can vary greatly depending on the species, typically measuring 3-4 µm in diameter, although some yeasts
can reach over 40 µm.
The yeast species Saccharomyces cerevisiae has been used in baking and fermenting alcoholic beverages for
thousands of years. It is also extremely important as a model organism in modern cell biology research, and is the
most thoroughly researched eukaryotic microorganism. Researchers have used it to gather information into the
biology of the eukaryotic cell and ultimately human biology. Other species of yeast, such as Candida albicans, are
opportunistic pathogens and can cause infection in humans.
2
Pasteur’s study on fermentation:
Louis Pasteur (1822-1895) was one of the most extraordinary scientists in history, leaving a
legacy of scientific contributions which include an understanding of how microorganisms carry
on the biochemical process of fermentation, the establishment of the causal relationship between
microorganisms and disease, and the concept of destroying microorganisms to halt the
transmission of communicable disease. These achievements led him to be called the founder of
modern microbiology.
After his early education Pasteur went to Paris, studied at the Sorbonne, then began teaching
chemistry while still a student. After being appointed chemistry professor at a new university in
Lille, France, Pasteur began work on yeast cells and showed how they produce alcohol and
carbon dioxide from sugar during the process of fermentation. Fermentation is a form of cellular
respiration carried on by yeast cells; a way of getting energy for cells when there is no oxygen
present. Pasteur found that fermentation could take place only when living yeast cells were
present.
Pasteur was then called upon to tackle one of the most persistent problems plaguing the French
beverage industry at the time, that of spoilage. Of special concern was the spoiling of wine and
beer, which caused both great economic loss to the industry and tarnished France’s reputation for
fine vintage wines. Vintners wanted to know the cause of l’amer, a condition that was
destroying the best burgundies.
Pasteur examined wine under the microscope and noticed that when aged properly the liquid
contained few spherical yeast cells. But when the wine turned sour, there was a proliferation of
bacterial cells which were producing lactic acid. It was the run-away production of lactic acid
that caused the spoilage. Pasteur suggested that gradually heating the wine to a temperature
range of 120 - 130 degrees Fahrenheit would kill the bacteria that produced lactic acid and allow
the wine to age properly. Pasteur’s book, Etudes sur le Vin, published in 1866 revolutionized the
wine industry.
In his work with yeast, Pasteur also found that air should be kept from fermenting wine. In the
presence of oxygen, yeasts and bacteria break down alcohol into acetic acid - vinegar. Pasteur
conducted many experiments with yeast. Pasteur showed that fermentation can take place
without oxygen (anaerobic conditions), but that the process still involved living micro-organisms
such as yeast.
Pasteur’s discoveries of the spoilage inherent in the natural fermentation process allowed him to
develop the fundamental concept of the “germ” theory of disease transmission. While
performing his experiments dealing with yeasts, and later with the silk-worm industry, Pasteur
also developed what has come to be known today as sterile technique, or the boiling or heating of
instruments and food to prevent the proliferation of microorganisms. Pasteur’s theories of
transmission of micro-organisms were gradually accepted by medical science during the decade
1870 - 1880, after work by noted British medical doctor and surgeon Joseph Lister confirmed the
3

germ-transmission theory of disease control in relation to infection rates in sterile and non-
sterile operating settings.
In 1897, scientist Edward Buchner reported that yeasts could be broken up and that the cell-free
yeast juice could ferment sugar. Later, it was found that the yeast juice contains the enzymes
necessary for the conversion of sugars to alcohol and carbon dioxide. As a consequence of
isolating the enzymes necessary for fermentation, mass production of beer and wine products
was greatly facilitated.
The basic understanding of the potential effects of naturally occurring yeasts and other
microorganisms and the subsequent collection, preservation, and testing of blood samples cannot
be overstated. As will be explained later in this material, any naturally occurring yeast or micro-
organism present during the collection phase of the whole blood sample may have a significant
effect on the resulting reported blood alcohol concentration.
ETHANOL IN BEVERAGES
Fermented Beverages: Wine ethanol concentrations generally range from 10-12% from the
fermentation of crushed grapes, but may be “fortified” by the introduction of additional alcohol
during the production process. Most table wine sold in the state of Alabama is 12.5% ethanol by
volume
3
. Most commonly, beer with a 3.2-5% ethanol concentration is sold at retail outlets
within the state. Beer ethanol concentrations when fermented can range from 3% to as high as
15%, but are regulated by state law to not exceed 13.9% alcohol by volume
4
.
Distilled Beverages: Production of distilled alcoholic beverages begins with the fermentation of
one or more natural grains such as corn, wheat, rye, or barley. These grains are the source of
carbohydrates (sugars) necessary for the process. The result is a wort (fermented fluid)
containing up to 12% ethanol by volume, which is then distilled by heating. Alcohol (ethanol),
which evaporates at 78 degrees Celsius, travels into a cooling apparatus (condenser) where it re-
liquefies. The now-concentrated ethanol can be collected in a storage container, and given
flavorings. Whiskey, vodka, gin, and a variety of other alcohol beverages are produced in this
manner. What distinguishes the various beverage types is the carbohydrate source (grain).
3
The strength of alcoholic beverages is most often shown as the percentage of alcohol by volume (sometimes shown
as % v/v or % ABV). This is not the same as the percentage of alcohol by weight (% w/v) since alcohol is less
dense than water: 5% v/v alcohol = 3.96% by weight (w/v); 10% v/v = 7.93% w/v and 40% v/v = 31.7% w/v.
4
See, Code of Alabama, 1975, section 28-3-1(3) defining “beer” as any fermented malt liquor containing one-half of
one percent or more of alcohol by volume and not in excess of 13.9 percent alcohol by volume, and defining “wine”
as either “fortified wine” having not more than 24 percent alcohol by volume, while “table wine” is defined as any
wine containing not more than 16. 5 percent alcohol by volume. [Note: Acts of 2009, No. 09-509, amended the
previous “14.9 percent” and replaced it with “16.5 percent” for the maximum alcohol content in table wine.]
In 2010, Act No. 10-607, amended the previous alcohol limitation on beer from the previous maximum amount by
volume of not in excess of five percent by weight and six percent by volume to include “high alcohol” beer products
for legal sale in Alabama, but retained the following requirement: “Beer or malt or brewed beverages sold by the
holder of a retail beer license for off-premises consumption …. [limited to] containing one-half of one percent or
more of alcohol or by volume and not in excess of five percent alcohol by weight and six percent by volume…”
4

Homemade distilled ethanol, commonly referred to as “moonshine”, while generally having no
flavoring added, possesses a fruit-like odor. The ethanol concentration in “moonshine” can range
from the low 60-proof range (30% ethanol) to as high as 120-proof (60% ethanol). The name
“moonshine” is derived from the nocturnal, clandestine nature of this illicit beverage production
5
.
Schematic of whiskey “Still” as used in production of “moonshine” whiskey:
ETHANOL IN BLOOD
Ethanol is classified as a ‘Central Nervous System’ depressant (CNS) whose impairing effects
are in proportion to its presence in the CNS. However, blood rather than brain tissue is the
preferred representative specimen for a chemical test for impairment because blood delivers
ethanol to and from the CNS and thereby is a reflection of CNS exposure to ethanol. A large
body of research exists which relates ethanol concentrations in whole blood with human
5
There are a number of statutes regulating or prohibiting the illegal manufacture of alcoholic beverages. Code of
Alabama, 1975, section 28-1-1, makes it “…unlawful for any person, firm, or corporation to have in his or its
possession any still or apparatus to be used for the manufacture of any alcoholic beverage of any kind…” Code
section 28-4-2 creates the offense of possession of illegal alcoholic beverages, with the penalty being an unclassified
misdemeanor. By Acts of 1915, the manufacture of illicit alcoholic beverages was made a misdemeanor, and by Acts
of 1919, the manufacture of prohibited liquors became a felony. [Limited by statute to a term of one to five years
imprisonment. See, Code section 28-4-24.]
The statute creating the crime of having possession of a still was adopted on September 30, 1919 with an effective
date of November 30, 1919. See, Code section 28-4-50: Unlawful possession of any still or device used to
manufacture any prohibited liquor or beverage. Code section 28-3A-25(9) makes it a misdemeanor offense for any
person to manufacture, transport, or import any alcoholic beverage into this state except by authorization of the ABC
Board.
5
performance. While any biological specimen may be analyzed for ethanol (blood, plasma,
serum, urine, saliva, sweat, ocular fluid, tissues), results for whole blood provide an accepted,
uniform standard for interpretations. For these reasons, statutes typically base per se limits for
ethanol content in whole blood (or breath, which is a related, but different, subject and is not
addressed in this publication). Forensic ethanol analyses are conducted with whole blood.
Determining a subject’s blood alcohol concentration (BAC) is the single most important issue
in establishing criminal and civil liability in a judicial proceeding where alcohol is alleged to
have been an element of the offense or the cause of action.
Absorption Principles: While the entire gastro-intestinal tract (GI) is capable of alcohol
absorption, almost 90% takes place in the small intestine where structural microvilli greatly
increase the surface area of the gut available for absorption. With its small molecular size,
ethanol readily crosses the GI tract membranes via passive diffusion and enters the circulation,
mixing completely with the fluid portion of blood. One of the fundamental concepts in
understanding blood-alcohol analysis is the fact that blood is approximately 85% water. Ethanol
distributes throughout the body where it rapidly crosses back through membranes into the tissues
and, most significantly, across the blood-brain barrier.
Blood: The adult human contains approximately five liters of blood, constituting about 8% of
the total body weight. Whole blood is a complex, heterogeneous mixture of solid material and
fluid. The solid material comprises red blood cells (erythrocytes), platelets (thrombocytes), and
white blood cells (leucocytes - lymphocytes and phagocytes). Each cell type has a specific
function:
• Red blood cells contain hemoglobin which binds oxygen and transports it throughout the body.
• Platelets participate in forming blood clots
• White blood cells are responsible for cell-mediated immune responses to foreign organisms
There are approximately 500 times more erythrocytes than leukocytes. The volume portion of
whole blood occupied by red cells is the hematocrit (HCT), which is defined as the volume of
red cells divided by the total blood volume. An average HCT for adult males is 40% to 50% and
for adult females, 35% to 45%. The HCT changes with age. A low HCT indicates a relatively
lower content of red blood cells in whole blood, which may be due to anemia, blood loss
(internal or external) or other disease conditions.
The fluid portion of whole blood prepared by removing the cellular solids from the anti-
coagulated, unclotted blood (typically by centrifugation) is called plasma. Serum is the fluid
portion of whole blood remaining after the blood has clotted and the clot is removed (typically
by centrifugation). Because plasma and serum contain no cellular solids, they contain a
relatively greater content of water than does whole blood. This is significant because ethanol
distributes into the various body compartments in proportion to their water content. In that
regard, plasma and serum, with a water content of 95% to 97%, will contain more ethanol than
the whole blood from which they are derived; whole blood being approximately 85% water.
6
This difference, 10% to 15%, highlights the importance of establishing what specimen - whole
blood, serum, or plasma - was tested for ethanol before making any interpretations of the results.
This issue will be discussed further in this publication.
Blood alcohol concentration (BAC): Results of forensic analyses are typically expressed as a
grams of ethanol per 100 mL of specimen or grams percent (g %) or simply percent (%). That a
blood ethanol concentration was reported to be 0.080 g/100 mL, however, does not imply that
100 mL of blood was analyzed and 0.080 grams of ethanol were detected. The Alabama
Department of Forensic Sciences (ADFS) analyzes 100 microliters (0.10 mL or 100 millionths of
a liter) of specimen. From this volume of specimen, the actual mass of ethanol detected is on the
order of 500 nanograms (500 billionths of a gram).
ETHANOL IN THE BRAIN
Alcohol affects various centers in the brain, both higher and lower order:
Ethanol is classified as a ‘Central Nervous System’ depressant (CNS), and affects the brain and
nervous system quickly after it enters the blood stream. The effects of ethanol are continuous
and progressive, meaning the overall effect on the CNS and on subject performance increases as
the concentration of ethanol in the CNS increases. However, all centers of the brain are not
equally affected by the same BAC - the higher-order centers are more sensitive than the lower-
7
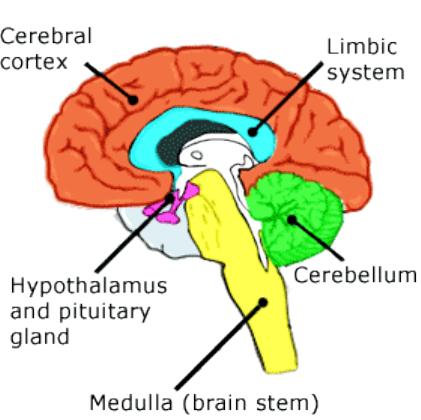
order centers. As the BAC increases, more and more centers of the brain are depressed until all
centers are depressed. The order in which alcohol affects the various brain centers is as follows:
Cerebral cortex
Limbic system
Cerebellum
Hypothalamus and pituitary gland
Medulla (brain stem)
Cerebral Cortex
The cerebral cortex is the part of the brain responsible for the highest functions of human
performance. The cortex processes information from the senses, performs “thought” processing
and consciousness (in combination with a structure called the basal ganglia), initiates most
voluntary muscle movements and influences lower-order brain centers. In the cortex, the effects
of alcohol are commonly recognized:
• Depresses the behavioral inhibitory centers - The person becomes more talkative, more self-
confident and less socially inhibited.
• Impedes the processing of information from the senses - Vision can be affected at low levels of
alcohol. Depth-of-field and peripheral vision are affected at BAC levels as low as 0.03% to
0.04%. General reflex response is slowed and fine motor skills are impaired at low levels of
alcohol. Also, the threshold for perception of pain is raised.
• Inhibits thought process - The person does not use good judgment or think clearly. These
effects become more pronounced as the blood alcohol concentration increases.
Limbic System
The limbic system consists of areas of the brain called the hippocampus and septal area. The
limbic system controls emotions, learning and memory. As alcohol affects this system, the
person is subject to exaggerated states of emotion (anger, aggressiveness, withdrawal) and
memory loss.
8

Cerebellum
The cerebellum coordinates the movement of muscles. The brain impulses that begin muscle
movement originate in the motor centers of the cerebral cortex and travel through the medulla
and spinal cord to the muscles. As the nerve signals pass through the medulla, they are
influenced by nerve impulses from the cerebellum. The cerebellum controls fine movements.
For example, a sober individual can normally touch finger to their nose in one smooth motion
with their eyes closed; if the cerebellum is not functioning, the motion would be shaky or jerky.
As alcohol affects the cerebellum, muscle movements become uncoordinated
6
. At the
approximate level of .08% to .10% blood alcohol concentration noticeable impairment can be
determined through the use of properly administered field sobriety tests. In addition to
coordinating voluntary muscle movements, the cerebellum also coordinates the fine muscle
movements involved in maintaining balance. As alcohol affects the cerebellum, a person
frequently loses his or her balance. At this stage, this person might be described as “falling down
drunk.”
Hypothalamus and Pituitary Gland
The hypothalamus is an area of the brain that controls and influences many automatic functions
of the brain through actions on the medulla, and coordinates many chemical or endocrine
functions (secretions of sex, thyroid and growth hormones) through chemical and nerve impulse
actions on the pituitary gland. The hypothalamus is also referred to as the “thermostat” of the
body and controls body temperature. Alcohol intoxication sufficient to depress the hypothalamus
will lower the body temperature.
Medulla
The medulla (or brain stem) controls or influences involuntary functions such as breathing, heart
rate, and consciousness. As alcohol depresses upper centers in the medulla, such as the reticular
6
Field Sobriety Tests, or FSTs, are “divided attention” tests that require both physical coordination and the ability to
process information simultaneously. Prior to the 1977 foundational study of field sobriety tests by Burns and
Moscowitz of the Southern California Research Institute (SCRI), the Traffic Institute at Northwestern University had
surveyed common sobriety tests then in use among law enforcement and prepared the “Alcohol Influence Report”
form with administration of common tests of sobriety such as the “walk the line” test, “pick-up-the coins test” and
the “finger to nose” test. However, research was not undertaken by the Traffic Institute to validate the relationship
between alcohol impairment and ability or inability to complete the aforementioned field tests.
The SCRI field research was conducted by four large police agencies over a period of two years and involving
thousands of subjects validated the use of three “standard” field sobriety tests: horizontal gaze nystagmus, the thirty
second one-leg stand, and the nine step “walk and turn.” The first test- horizontal gaze nystagmus- is not a divided
attention test, but the observation of the involuntary movement of the eye while following a stimulus. The 1981 final
report validated the three test battery to a correlation of .77 (1.00 being perfect correlation) when all three tests were
used to evaluate a subject at .10% BAC or greater.
9
formation, a person will start to feel sleepy and may eventually become unconscious as BAC
increases. If the BAC gets high enough to influence the breathing and heart rate, a person will
breathe slowly or stop breathing altogether, and concurrently blood pressure will fall. These
conditions can be fatal.
Stages of Alcoholic Influence/Intoxication: Kurt M. Dubowski, Ph.D., University of Oklahoma
Department of Medicine, a noted authority on alcohol and the dynamics of ethanol distribution
and the effects on the human body, developed a chart describing the clinical signs and symptoms
resulting from the ingestion of alcohol and which is based on the blood alcohol concentration
measured in grams/100 mL. Because not all centers of the brain are affected at the same blood
alcohol concentrations, different subject behaviors may be visible at similar alcohol levels. This
gives rise to the myth that “everyone reacts differently to alcohol.” Actually, everyone reacts the
same to alcohol; their CNS becomes depressed. What is different, however, is the degree to
which each function of the CNS is depressed in each subject. The sum of these depressed
functions results in the behaviors visible among subjects, which may be different. The fact that
blood alcohol concentrations overlap for each clinical sign demonstrates this phenomenon.
CLINICAL SIGNS/SYMPTOMS
0.01-0.05 Subclinical
Influence/effects usually not apparent or obvious
Behavior nearly normal by ordinary observation
Impairment detectable by special tests
0.03-0.12 Euphoria
Mild euphoria, sociability, talkativeness
Increased self-confidence; decreased inhibitions
Diminished attention, judgment and control
Some sensory-motor impairment
Slowed information processing
Loss of efficiency in critical performance tests
0.09-0.25 Excitement
Emotional instability; loss of critical judgment
Impairment of perception, memory and comprehension
Decreased sensatory response; increased reaction time
Reduced visual acuity & peripheral vision; and slow glare recovery
Sensory-motor in-coordination; impaired balance; slurred speech; vomiting; drowsiness
10
0.18-0.30 Confusion
Disorientation, mental confusion; vertigo; dysphoria
Exaggerated emotional states (fear, rage, grief, etc)
Disturbances of vision (diplopia, etc.) and perception of color, form, motion, dimensions
Increased pain threshold
Increased muscular incoordination; staggering gait; ataxia
Apathy, lethargy
0.25-0.40 Stupor
General inertia; approaching loss of motor functions
Markedly decreased response to stimuli
Marked muscular incoordination; inability to stand or walk
Vomiting; incontinence of urine and feces
Impaired consciousness; sleep or stupor
0.35-0.50 Coma
Complete unconsciousness; coma; anesthesia
Depressed or abolished reflexes
Subnormal temperature
Impairment of circulation and respiration
Possible death
0.45+ Death
Probable death from respiratory arrest
11
BASIC PRINCIPLES OF BLOOD ALCOHOL ANALYSIS
Gas Chromatography
The oldest and most fundamental chemical test for intoxication is a test for ethanol in blood.
Blood-alcohol analysis is commonly performed in driving under the influence (DUI) arrests and
investigations of serious injury or fatal traffic accidents. This analysis is undertaken with whole
blood samples collected from the suspect, as well as from any deceased driver or passenger.
There are a variety of laboratory methods to determine the alcohol concentration in a biological
specimen. However, the criminal justice practitioner should be familiar with the two most
common methods: gas chromatography and enzymatic assay.
In General: Forensic ethanol analyses typically employ a scientific process known as gas
chromatography, which is a widely-used technique in modern analytical chemistry. Known as a
separation science, gas chromatography is an instrument-based technology that separates
mixtures of molecules based upon their chemical and/or physical properties. The instrument is
called a gas chromatograph (commonly abbreviated as GC). Components of all GCs include an
injection device for introducing the sample mixture to the stationary medium, a stationary
medium contained within the column enclosed in a temperature-controlled oven where the actual
separation takes place, a carrier gas to move molecules through the stationary medium, and a
device to detect the separated molecules. These components are connected in series to create a
closed, tubular pathway for the molecules and gas to travel through the system.
GC Operation: Separations occur with molecules in the gas state, which requires that most
substances be vaporized during the analysis. This is accomplished by “injecting” via syringe a
mixture of the molecules into a very hot (140°C to 250°C), glass-lined chamber where the
molecules are vaporized into the necessary gas state. Pressurized carrier gas (typically helium)
flows through this chamber and carries the vaporized molecules to a stationary, porous, inert
powder such as silica packed into a long narrow stainless steel tube or glass column. This
packed material is called the stationary phase because it remains stationary within the column as
the molecules are carried through by the pressurized inert gas stream. The vaporized molecules
“stick” to the stationary phase based upon their chemical or physical attraction in a process
called adsorption. The column is then heated (hence the oven) to a temperature where molecules
begin to “boil away” or dissociate from the stationary phase to be carried downstream by the gas
(called the moving phase) in a process called elution.
As these molecules flow downstream, they actually undergo repeated cycles of re-adsorption and
dissociation with the stationary phase in a process called partitioning. The stronger the attraction
is between molecules and the stationary phase, the more frequently they re-adsorb and remain
“stuck” and the slower they will elute from the column. This is not unlike people stepping on
and off a moving sidewalk, where the speed of travel depends upon the time spent on the moving
versus stationary platforms. A current alternative stationary phase to the solid porous powder
packing is a waxy or resinous coating applied to the inner surface of a long coil of flexible glass
capillary tubing. This coating serves the same purpose as the solid porous powder packing by
12

offering a surface to which molecules may repeatedly adsorb and dissociate as they flow through
the column. In a properly designed system, the end result of partitioning is the elution of a
succession of molecules separated into groups with similar or identical chemical and/or physical
properties and, hence, structure.
Eluted molecules leave the column and flow into a detection device. The device typically used
for ethanol analyses is the flame-ionization detector (FID), which generates an electrical signal in
proportion to the mass of molecules passing through that are combusted and the ionized form
detected. The successive waves of separated molecules eluting from the column and passing
through the detector provide a time chart (called a chromatogram) which appears as a series of
Gaussian (bell-shaped) peaks, each representing a group of eluted molecules. The time taken by
each group of molecules to elute is called the retention time and is an identifiable characteristic
of the molecules.
Individual substances may be quantified by measuring the size of the peaks eluting with the
retention time characteristic for the substance. The GC is calibrated with a series of samples or
calibrators containing known amounts of substances and establishing the mathematical detector
responses for each substance separated in the mixture. This response is then used with linear
regression mathematics to calculate the mass of each eluted substance. This is called the
calibration curve.
Headspace Gas Chromatography
Ethanol is a small molecule that readily evaporates into a gas state at ambient temperature, even
from solution in water. This volatility lends ethanol to a special type of analysis called
headspace GC, which is a process well suited for the analysis of gases. Headspace analysis
refers to the analysis of the air (head) space above a liquid or solid in a container. This is an
indirect analysis because the vapors emitted from the sample are tested rather than the sample
itself. Headspace GC differs from conventional GC in that a vapor mixture rather than a liquid
sample is introduced into the GC. Similarly, volatile substances such as methanol, acetone and
2-propanol (isopropanol) may also be separated and measured with headspace GC.
13

The contents of a liquid’s headspace reflects the contents of the liquid itself. A fundamental
principle of science is Henry’s Law (1803) which states: “At a constant temperature, the amount
of a given gas dissolved in a given type and volume of liquid is directly proportional to the
partial pressure of that gas in equilibrium with that liquid.” In other words, in a sealed vessel
and at equilibrium, volatile substances will be present in the vapor state above a liquid at
concentrations in proportion to their respective concentrations in the liquid. Therefore, if a
specimen is placed in a sealed vial, one may then determine the concentration of a volatile
substance in the liquid by analyzing the equilibrated headspace above the liquid. With headspace
GC, only volatile substances are analyzed so the potential universe of interferences is drastically
limited. Further, because the non-volatile substances remaining in the specimen are not injected
into the GC, the longevity of the GC column is extended and necessary maintenance reduced.
Because of this factor, however, some crime laboratories will dedicate a GC solely to analysis of
ethanol and attempt to operate the system without conducting periodic checks on the
maintenance or repair of the GC.
Specimens are prepared for headspace GC analysis by dispensing a small volume of whole blood
(typically 100 µL) into a glass vial (typically 20 mL) and adding a diluent (typically 1.0 mL of a
saturated sodium fluoride solution containing 1-propanol or tert-butanol as internal standard).
Vials are sealed by crimp-cap and placed into a carousel that holds many vials for a single run.
The carousel is part of an autosampler that attaches to the GC and automatically samples
multiple vials in sequence. (Please refer to Appendix A.)
The sealed vials are gently heated and mixed at 40°C to 70°C for 5 minutes to 20 minutes.
During this period, volatile substances in the liquid equilibrate with the headspace in the vial
above the liquid. The vial is then pressurized with carrier gas, after which the gas flow is
reversed so that the pressurized vapor in the vial may flow to the column through a transfer line.
The process of sample equilibration, mixing and transfer, and GC analysis is automated so the
analysis may proceed unattended. Specimens are typically analyzed in batches along with
quality control samples to allow monitoring of the accuracy and precision of the process.
Example of sealed vial used in GC testing:
14

The diluent used in sample preparation is vital to the accuracy and reliability of headspace GC.
First, the internal standard, a substance of similar chemical and physical properties as the target
analyte, provides a retention time marker and a scale against which the quantity of the substance
is normalized. This is not unlike a ruler in a photograph providing a reference for object size.
Second, the saturated salt, typically sodium fluoride, is added to promote volatilization of
substances from the liquid specimen, to increase the sensitivity of the analysis. This can
introduce variability to the reported ethanol concentration if the calibrators used to prepare the
calibration curve are not made in whole blood prepared in an identical manner to that used in
collecting the forensic specimens.
A recent innovation in blood alcohol analysis involves splitting the injected specimen vapor into
two parallel capillary columns with somewhat different stationary phases and in which target
substances are expected to elute with somewhat different retention times
7
. Assurance that
ethanol is correctly identified and quantified is improved because the eluted peak identified as
ethanol must meet the characteristic retention times for both columns in the same analysis.
Whereas use of dual-capillary column headspace GC improves confidence in results, it does not
render obsolete single-column analyses
8
.
Reading the Chromatogram: The chromatogram is a graph that shows when each molecule
makes contact with the detector at the end of the column. Since different kinds of molecules will
reach the detector to be burned (ionized) at different rates depending on their size, shape, and
other properties, the graph produced will have a series of peaks that must be read by the lab
analyst. The retention time is the length of time it takes the separated compound to go from the
injection to the detector through the gas chromatograph. The chromatogram will ideally show
sharp, symmetrical peaks at different points in time representing the different kinds of molecules
emerging from the column. (Please refer to Appendix B.)
The time it takes for a peak to appear in the known samples of ethanol is then compared with the
chromatogram for the unknown sample. If a peak appears in the unknown sample at the same
time that the peak appears in the known ethanol sample, then the unknown blood sample
likewise contains ethanol.
It is important to note that the area under the peak represents the concentration. How the lab
analyst determines the area is crucial to the end result as more area represents a higher ethanol
concentration. The baseline is critical in the calculation of the area as it is the boundary of that
7
Dual column chromatography utilizes a ‘Y’ splitter to take the single sample from the sealed vial and to “split” the
sample into two GC columns, thus allowing the scientifically approved two test analysis of the single unknown
sample. For more information on GC columns, see the various GC products produced by Restek chromatography at:
www.restek.com
8
http://las.perkinelmer.com/Catalog/ProductInfoPage.htm?ProductID=BAANALYSIS
http://las.perkinelmer.com/Content/RelatedMaterials/CaseStudies/CST_GasChromaIncrAccuracyBloodAlchlAnaly.
pdf
15

measurement. (Please refer to Appendix D.) As stated previously in this publication, determining
a subject’s blood alcohol concentration (BAC) is the single most important issue in establishing
criminal and civil liability in a judicial proceeding where alcohol is alleged to have been an
element of the offense or the cause of action.
The area of the peak is then compared to at least three known ethanol standards which are plotted
on a graph in what is called a calibration curve
9
. If the known ethanol standard is a sample with .
08% ethanol and the second known standard is .16% ethanol, and the third standard is .32%
ethanol, then the area of the peak from the suspect’s blood sample is measured against the
calibration curve from the three known standards to determine the suspect’s blood alcohol
concentration.
Possible Problems with Gas Chromatographs:
Problem Cause Solution
Peak has a flat top Chromatogram is off scale Sample must be diluted and retested
Peak slants System was overloaded Sample must be diluted and retested
Peak has a shoulder Dirty column, or co-eluting compound Change column and/or retest sample
Two peaks are together Another compound has similar retention time Change oven temperature or gas flow
Peak is very broad Dirty inlet or column Change inlet and /or column
Ghost peaks Dirty column Change column
Carry-over Dirty inlet or column Change inlet and/or column
(Please refer to Appendix F showing examples of chart irregularities.)
Hospital Analysis: Clinical and hospital laboratories also conduct ethanol determinations but
typically do so with serum rather than whole blood. This is because clinical laboratories are
engaged in diagnostic testing, which is focused primarily on a vast universe of substances in
serum. Ethanol is simply an additional analyte for testing by use of existing instrumentation. As
contrasted to forensic lab testing where the GC is used, hospital and clinical laboratories use the
enzymatic method to distinguish and quantify ethanol in serum. The enzymatic method is the
most common chemical process in hospital laboratories. The main purpose of utilizing the
enzymatic method rather than GC is to obtain the quickest result possible. A GC run may
require up to eight hours while the enzymatic method can be accomplished in as short a time as
20 minutes. However, the enzymatic methods lack the exactness (accuracy) of the GC method,
with an average of 10% – 20% deviation common in analysis, as well as a lack of specificity for
isopropyl and butyl alcohols.
9
See, Erwin, Defense of Drunk Driving Cases: Civil/Criminal at section 17.08 (Matthew Bender, 3
rd
Ed. 2007)
16

Using the enzymatic method, alcohol dehyrogenase (ADH) is an enzyme which is used to
measure the concentration of alcohol in biological specimens. In the reaction, alcohol is oxidized
to acetaldehyde by ADH in the presence of a coenzyme, nicotinamide adenine dinucleotide
(NAD), which is reduced to NADH. [Ethanol + NAD = Acetaldehyde + NADH + H]
10
Another difference is that clinical laboratories typically express ethanol results as milligrams of
ethanol per deciliter of specimen (mg/dL). This difference, however, reflects only a difference in
the units of expression and not the actual content of the specimen.
Most importantly, while it is not forensically recommended to use hospital or clinical results for
evidentiary purposes, if such results are employed, the impact of the different methodologies and
specimen on the interpretation of the result must be examined. Blood specimens drawn in a
hospital setting may produce false negative results with enzyme assays. As example, Ringer’s
lactate solution is typically used for fluid replacement and blood volume expander. Ringer’s
lactate contains lactic acid which will react with ADH analogous to ethanol thereby producing a
false positive ethanol finding. Diabetics typically have acetone and isopropyl alcohol in their
blood and the enzymatic test will determine both ethanol and isopropyl alcohol given an apparent
BAC greater than the true value of ethanol in the blood specimen.
10
Enzymatic testing is actually the measurement of NADH, one of the enzymes used in oxidizing the alcohol to
acetaldehyde, and not a measurement of ethyl alcohol itself. Gas chromatography, by contrast, is a whole blood
measuring test. Gas chromatography is preferred for the analysis of ethanol because, among many other advantages,
it employs separation technology to discriminate the target analyte.
17

ALABAMA LAW ON CHAIN OF CUSTODY OF BLOOD SAMPLES
Who Can Draw Blood?
Under the Code of Alabama, 1975, section 32-5A-194 (a)(2), “only a physician or a registered
nurse (or other qualified person)” is authorized to take a blood sample for use as evidence in civil
and criminal cases. See, McGough v. Slaughter, 395 So. 2d 972 (Ala. 1981). The Court of Civil
Appeals held in Lankford v. Redwing Carriers, Inc., 344 So. 2d 515 (Ala. Civ. App. 1977) the
purpose of allowing only physicians, registered nurses, or duly licensed clinical laboratory
technicians to withdraw blood samples is to ensure that standardized procedures and equipment
is used, thereby preserving the validity of the test. “Strict compliance with the Chemical Test for
Intoxication Act is required.” Lankford, supra.
Alabama Code section 32-5A-194 (a)(2) mandates that only certain licensed persons may draw
blood samples. By statute, all licensed physicians and registered nurses are presumed competent
and qualified to draw evidentiary blood samples. The term “other qualified person” is not further
defined within the Code, but several prior court decisions held that a licensed tab technologist is
qualified to draw blood for evidence and subsequent analysis. See, McGough v. Slaughter, 395
So. 2d 972, 975; Rehling v. Carr, 330 So. 2d 423 (Ala. 1976); and Powell v. State, (515 So. 2d
140 (Ala. Cr. App. 1986). However, an EMT or an EMT-paramedic is not authorized to draw
blood for evidentiary purposes
11
. It is the policy of the Alabama Department of Public Health,
EMS Division, that emergency medical technicians, including EMT-paramedics, are not
authorized to draw blood for non-therapeutic reasons, such as obtaining evidence for law
enforcement officers. According to the Alabama Department of Public Health, the only
permissible reason for an EMT to draw blood is for medical intervention and only at the specific
direction of a medical provider.
In Powell v. State, supra., the defendant submitted to a blood sample drawn by a licensed
medical laboratory technician. The sample was obtained under clinical conditions. Defense
counsel later objected to the blood draw, but the Court specifically held the lab technician “was
therefore qualified to draw blood samples” in accordance with the statute. Powell, 515 So. 2d at
1446. In the later case of Ingram v. State, 720 So. 2d 1036, 1041 (Ala. Cr. App. 1998), where the
11
In August 2007, the Alabama Department of Public Health issued an official opinion prepared by the Compliance
Coordinator for the Office of Emergency Medical Services and Trauma stating: “The ADPH legal department
advised that this procedure” … [drawing blood by EMT’s at the scene of an accident at the request of law
enforcement officials] … “could not be performed on individuals that did not require medical interventions by on
scene Paramedics. In this instance, the Paramedics would be exceeding their scope of license and would be in
violation of State EMS Rules.”
In August 2010, the Chief of the Highway Patrol Division of the Department of Public Safety, Major Charles
Andrews, issued a Memorandum to all arresting officers of the Highway Patrol Division which stated: “It has
recently been brought to the attention of the Division Chief that some troopers are requesting EMS Personnel (i.e.
Emergency Medical Technicians, Paramedics, etc.) to draw blood for purposes related to an individual who is
suspected/charged with driving under the influence. Such practice is not acceptable and shall discontinue
immediately.”
18
blood sample was drawn by a licensed medical technologist working as a medical laboratory
technician, no objection was made to the technician’s credentials or qualifications
12
.
It is instructive to note that all of the above cited cases, except Ingram, were decided prior to the
comprehensive revision of the pre-existing statute to the current 32- 5A-194, commonly known
as the “Chemical Test for Intoxication Act.” The original statute was enacted in 1969 and was
codified at Title 36, section 155. The original statute was worded more exactly than the current
statute. In the prior Title 36, section 155, in paragraph (C), the statute stated the following:
“Only a physician, registered nurse, or duly licensed clinical laboratory
technologist or clinical laboratory technician acting at the request of a law
enforcement officer may withdraw blood for the purpose of determining the
alcoholic content therein.”
The current Code section was enacted in 1988. Upon revision, concerning the appropriate
persons authorized to draw blood samples, the revised statute retained the terms “physician” and
“registered nurse” but replaced “licensed clinical laboratory technologist” and “clinical
laboratory technician” with the words “other qualified person.” The term “other qualified
person” is not further statutorily defined
13
. Presumably, the Alabama Department of Forensic
Sciences has the authority under the Alabama Administrative Code to determine appropriate
qualifications or set standards for credentialing for persons to meet the term “other qualified
person,” but as of this publication, DFS has not done so. Therefore, the term “other qualified
person” is left open to the sound discretion of the trial court to determine the proper training,
certification, and credentials of the individual that drew the blood sample.
Custody of the Sample:
By statute and decisional law, the state must identify the person and offer into evidence the
credentials of the duly authorized person who drew the blood sample from the defendant. The
blood sample cannot be presumed to have been taken in the correct manner unless the blood
draw is established by the person who took the sample. The law of blood test admissibility in
Alabama courts is extensive and clear: blood test evidence must be established by both predicate
and chain of custody. These two requirements are properly subject to thorough cross-
examination by defense counsel.
The leading Alabama case in this area regarding admissibility of the results of laboratory samples
is Ex parte Holton, 590 So.2d 918 (Ala. 1991) which examined in detail the theory of chain of
12
In Powell, the person drawing the blood sample, a Margaret Jackson, testified that she was a duly licensed
laboratory technician, certified by the American Medical Technologists Registry, the National Board. She further
testified that she was licensed by the National Registry and certified by the Alabama Association of Medical
Technicians (See, Code of Alabama, 1975, section 34-18-21). The Court held she was therefore “qualified to draw
blood samples in accordance with Code of Alabama, 1975, section 32- 5A-194(a)(2).” Powell was a pre-1988
decision.
13
See, Act 88-660 which transferred supervisory authority of the state’s implied consent testing program from the
State Board of Health to the Department of Forensic Sciences and re-wrote and revised the state’s Chemical Test for
Intoxication Act.
19

custody
14
. In order to establish a proper chain, the State must show to a reasonable probability
that the object is in the same conditions, and not substantially different from, its condition at the
commencement of the chain. The court requires that proof be shown on the record with regard to
exact chain of custody of the sample.
The chain of custody is composed of “links.” A link is anyone who handled the item. The State
must identify each link from the time the item was seized. In order to show a proper chain of
custody, the record must show each link and also the following with regard to each link’s
possession of the item: 1) the receipt of the item; 2) the ultimate disposition of the item, i.e.,
transfer, destruction, or retention; and 3) the safeguarding and handling of the item between
receipt and disposition. If the State, or any other proponent of demonstrative evidence, fails to
identify a link or fails to show for the record any one of the three criteria as to each link, the
result is a “missing” link, and the item is inadmissible. If, however, the State has shown each
link and has shown all three criteria as to each link, but has done so with circumstantial evidence,
as opposed to the direct testimony of the “link,” as to one or more criteria or as to one or more
links, the result is a “weak” link. When the link is “weak,” a question of credibility and weight is
presented, not one of admissibility. In this area, see also, Lee v. State, 748 So. 2d 904 (Ala. Cr.
App. 1999)
15
.
In regards to blood samples, all three Alabama appellate courts have adhered to the ‘link’
analysis for establishing the chain of custody. In Creel v. State, 618 So.2d 132 (Ala. Cr. App.
1992), a vehicular homicide case where chain of custody of the blood sample was questioned,
the Court found the state did not establish a chain of custody with respect to vials of blood drawn
from the defendant following an automobile accident. The transmittal forms accompanying vials
upon their arrival at Department Forensic Sciences in Auburn were not signed or initialed by
person who shipped blood from Dothan, and the forensic sciences investigator in Dothan who
collected blood from investigating officers and placed the vials in a refrigerator with the
transmittal forms could not unequivocally testify that he was person who shipped the blood vials.
The Courts generally apply a “reasonableness” test in regards to maintaining security over the
blood samples. The case of Wallace v. State, 574 So.2d 968 (Ala. Cr. App. 1990) is instructive.
In that case, the nurse on duty drew two blood samples at the hospital and handed two sealed
samples to the investigating police officer. The officer then placed the vials inside a sealed
Styrofoam box (referred to in the Court’s opinion as ‘a DUI evidence kit’) in a refrigerator at
City Hall where the kit remained over the weekend. The refrigerator was not locked or secured
14
Ex parte Holton was later cited for authority in Birge v. State, 973 So. 2d 1058 (Ala. Cr. App. 2007) for the
requirement that the proponent of the offered evidence must establish a strict chain of custody of samples collected
for forensic analysis. See, also, Swanstrom v. Teledyne Continental Motors, Inc., 43 So. 3d 564 (Ala. 2009): The
Alabama Supreme Court and the Court of Criminal Appeals have “consistently cited and relied on Ex parte Holton
for its statement of the principles establishing the legal requirements for proving a proper chain of custody.”
15
Lee was later modified by Pruitt v. State, 954 So. 2d 611 (Ala. Cr. App. 2006) regarding the issue of admissibility
of the state’s Certificate of Analysis, but not on the issue of demonstrating the need for the chain of custody.
20

and was accessible to any number of city employees. The following Monday morning, the
officer retrieved the still-sealed kit and delivered it to the forensics lab for analysis. The forensic
analyst testified that there was nothing to indicate the kit had been tampered. The Court found
the chain of custody of blood samples was sufficient despite evidence indicating some
carelessness in storage of the samples
16
.
The Court noted:
“Although the evidence indicates some carelessness in the storage of the blood samples,
we find that the evidence of the test results was properly admitted. ‘[I]t is presumed that
the integrity of evidence routinely handled by governmental officials was suitably
preserved “[unless the accused makes] a minimal showing of ill will, bad faith, evil
motivation, or some evidence tampering.” United States v. Roberts, 844 F. 2d 537, 549-50
(8th Cir.). Applying those principles to the facts of this case, we find that the State
proved to a reasonable probability that the blood samples were the same as, and not
substantially different from, the samples as they existed at the beginning of the chain. Ex
parte Williams, 548 So. 2d 518, 520 (Ala. 1989); Suttle v. State, 565 So. 2d 1197 (Ala.
Crim. App. 1990).”
Another example of circumstantial evidence to support the chain of custody requirement was
found in Bartlett v. State, 600 So. 2d 336 (Ala Cr. App. 1991), the appellant’s blood was drawn
by a hospital nurse and the blood sample vial shortly thereafter transported to the hospital
laboratory for analysis. The nurse drawing the blood labeled the vial with the appellant’s name
and placed the sample in a pre-vacuum sealed vial. The lab technician responsible for the
analysis testified that he would not have accepted the sample for analysis had it not been in a
sealed condition upon arrival at the hospital lab. The fact that a ward clerk transported the
sample to the laboratory for analysis did not defeat the chain of custody. In Bartlett, the Court
stated:
“To establish a sufficient predicate for admission into evidence it must be shown
that there was no break in the chain of custody. Identification and continuity of
possession must be sufficiently established to afford ample assurance of the
authenticity of the item. Ex parte Yarber, 375 So. 2d 1231, 1234 (Ala. 1979). ‘A
16
See a similar set of facts in Cook v. State, 52 Ala. App. 290 So. 2d 228 (Ala. Crim. App. 1974): The Court held
that the overnight storage of blood samples in a funeral home refrigerator was not a failure in the chain of custody
requirement. The funeral home employee admitted that he could not be sure that someone had not removed the
samples from the refrigerator, or handled them in some way, during the night. The funeral home employee did
testify that no one had tampered with the vials prior to delivery to the deputy sheriff who drove them the state
laboratory the following day.
See the following related cases: Powell v. State, 515 So. 2d 140 (Ala. Crim. App. 1986): Sample stored in
refrigerator in district attorney’s office for a period of two days prior to delivery to state laboratory ; Stone v. State,
641 So. 2d 293 (Ala. Crim. App. 1993): Because of the late hour at which the sample was drawn, the investigating
state trooper took the sealed sample home and stored the sample in his home refrigerator. In both cases, despite fact
that other persons had access to the refrigerator or storage compartment, that fact alone did not cause a fatal flaw in
the chain of custody.
21

party need not negative the remotest possibility of substitution, alteration or
tampering with the evidence.’” Whetstone v. State, 407 So.2d 854, 859 (Ala. Cr.
App. 1981).
Likewise in Moorman v. State, 574 So.2d 953 (Ala.Cr.App. 1990), the Court found the chain of
custody sufficient where, in prosecution for criminally negligent homicide following a fatal
automobile collision, the chain of custody for a blood sample taken from the defendant was
sufficiently established even though two “links” in the chain (the unit secretary at the hospital
who sent the sample to the laboratory and the person from the laboratory who picked up the
sample) did not testify
17
. The evidence was sufficient to establish chain of custody for victim’s
body, even though the person who transported the body to the morgue and the county coroner
who received the body did not testify.
However, in Suttle v. State, 565 So.2d 1197 (Ala. Cr. App. 1990), the chain of custody was not
established, and the blood sample was deemed inadmissible. The appellant’s conviction for
vehicular homicide was reversed because the state failed to account for the location of the blood
samples drawn from the defendant during the four days between the time the samples were taken
by the nurse and the time they were received by the state’s forensic expert. The nurse who gave
the blood samples to the trooper did not testify. The forensic analyst received the blood through
the U.S. mail. The toxicologist who received the samples could not testify where the samples
had been located during the previous four days. The court held it was reversible error to admit
test results conducted on a blood sample when there was an insufficient chain of custody for the
sample.
The importance of proving the chain of custody of a blood sample was demonstrated in Miller v.
State, 484 So. 2d 1203 (Ala. Cr. App. 1986) where the investigating state trooper in a traffic
fatality case secured blood samples from the defendant at the local hospital, then took the blood
sample vials to the Jacksonville state trooper office, “put it in the envelope, sealed it and initialed
it” then placed the sample in the department’s outgoing mail, not the U.S. mail. Three days later,
the sample was delivered to the Department of Forensic Science lab in Birmingham for analysis.
There was no accounting for the location or security of the blood samples for the three days prior
to delivery at the DFS lab.
Although the use of the U.S. Mail attaches a legal presumption that materials are delivered in
substantially the same condition as when placed in the mailbox or post office, no such
presumption is attached to “regular outgoing mail” delivery service used by a state agency. “To
establish a sufficient predicate for admission into evidence it must be shown that there was no
break in the chain of custody. ... Where ‘missing links’ are involved in the chain of custody the
17
See, as example, the case of Gothard v. State, 452 So. 2d 889 (Ala. Crim. App. 1984): the Court held that
conflicting testimony about when the specimens changed hands did not prevent the state from establishing a
sufficient chain of custody. The chain of custody rule provides that a party seeking to introduce into evidence results
of a laboratory analysis has the burden of proving that the specimen or object analyzed was, in fact, taken from the
particular person alleged. Despite the conflicting testimony of the difference in time when the specimen was
delivered, the state established to a reasonable certainty that there had been no substitution, alteration, or tampering
with the specimen.
22
question presented is one of admissibility rather than credibility.” Citing Whetstone v. State, 407
So. 2d 854, 859- 60 (Ala. Cr. App. 1981).
In the case of Green v. Alabama Power Company, 597 So. 2d 1325 (Ala. 1992), a wrongful death
case where the defense was contributory negligence on part of the decedent, fluid samples were
taken during the autopsy which, after analysis, allegedly showed the presence of a controlled
substance. The plaintiff objected to admissibility of the sample where the analysis of blood and
other body fluid samples were shipped by U.P.S. delivery service and subsequently analyzed at
the DFS laboratory.
In Green, the Alabama Supreme Court held:
“In chain-of-custody cases involving “specimens taken from the human body,”
the proponent of the evidence must demonstrate “where and by whom the
specimen was kept and through whose hands it passed.” J. Richardson, Modern
Scientific Evidence, 13.14a ( Ed. 1974). Gothard v. State, 452 So. 2d 889, 890
(Ala. Cr. App.), cert. striken, 450 So. 2d 479 (Ala. 1984).” Suttle v. State, 565 So.
2d 1197, 1199 (Ala. Cr. App. 1990) (reversing vehicular homicide conviction for
failure of prosecution to account for blood sample during four day interval
between delivery of unsealed sample to police officer and reception at
laboratory.)”
The Supreme Court held in Green that a similar four day gap between the date of the blood draw
and the subsequent delivery to the forensic laboratory, without explanation as to the sample’s
location or control, would render the sample inadmissible into evidence.
The case of Jones v. City of Summerdale, 677 So. 2d 1289 (Ala.Cr.App. 1996) is illustrative of
the requirement for live witness testimony to establish both the manner of the blood draw and
establishment of the chain of custody. In Jones, the Court of Criminal Appeals held conformity
with evidentiary predicate was required for the admission of blood tests as well as compliance
with chain of custody requirements.
The Jones case holds that results of a blood test administered to determine blood alcohol content
may be received into evidence, provided a proper predicate is laid. The state must first lay a
sufficient predicate in support of such evidence to indicate its reliability. A lab report indicating
the results of a blood alcohol test, without any supporting testimony, invites reversible error. In
Jones, the state did not present any testimony regarding the blood test performed on the
appellant. The Court of Criminal Appeals held if the State elects to offer the results of blood
alcohol test into evidence, the State must comply with the rules of evidence.
The Court’s opinion stated:
“In this case the state offered the blood test into evidence without any testimony
indicating the reliability of the test, who performed the test, or the circumstances
under which the test was performed. The trial court received the test without any
23

foundation whatsoever having been established. The trial court erred to reversal
when it incorrectly received the blood evidence into evidence. Jones v. City of
Summerdale, 677 So. 2d at 1291.
In the case of Nelson v. State, 551 So. 2d 1152 (Ala. Cr. App. 1989), citing the prior case of Kent
v. Singleton, 457 So. 2d 356 (Ala. 1984), the Court of Criminal Appeals held it fundamental to
establishing admissibility that blood evidence must demonstrate the chain of custody
requirement. Without establishing a strict chain of custody, the sample results are inadmissible
into evidence. The evidence in the Nelson case did not disclose the identity of the person who
withdrew the blood sample at the hospital. The trial court properly refused to admit the test
results under § 32-5A-194. The results were not admissible under general evidence principles as
there was no proof that the test performed on the defendant was conducted according to accepted
scientific methods and there was no proof of the qualifications of the person who withdrew the
blood sample. The Court further held the mere fact that the blood sample was taken at a hospital
does not insure its reliability
18
.
Finally, it should be noted that the Alabama Supreme Court has rejected the contention that
chain-of-custody requirements in a civil action should be less demanding than the requirements
in a criminal proceeding. In Swanstrom v. Teledyne Continental Motors, Inc, 43 So. 3d 564 (Ala.
2009), the Court summarily rejected the plaintiff’s argument that a chain of custody requirement
in a civil action are less strict than requirements in a criminal case. Where the toxicology report
had several missing links in the custody requirement, the toxicology report was presumptively
inadmissible into evidence
19
.
BLOOD SAMPLE COLLECTION
In General: The proper collection of a forensic blood sample to be analyzed for use as evidence
is the first critical step in establishing a proper chain of custody and most importantly to establish
18
See in general, Annotation, Necessity and Sufficiency of Proof that Tests of Blood Alcohol Concentration Were
Conducted in Conformance with Prescribed Methods, 96 A. L. R. 3d 745 (1979).
19
In the Swanstrom case, the Court noted the toxicology report lacked any information regarding the condition of the
blood samples upon receipt; whether the sample kit was sealed when received by the laboratory; who signed for and
accepted the samples at the laboratory; how the samples were stored prior to testing; the date, method, and types of
tests that were run on the samples; and the fact the samples were unaccounted for during the eight days between the
time they were collected and the time they arrived at the lab.
24

the sample’s integrity. Blood test evidence plays a significantly important role in determining
criminal culpability in a traffic assault or traffic homicide case. In a civil action, blood evidence
is likewise crucially important to determine liability where negligence is the underlying cause of
action. The blood evidence taken must be properly accounted for throughout every step in the
collection, storage and analysis process.
Sample Collection: Samples collected by law enforcement agencies for evidentiary purposes
are usually obtained by using a forensic blood collection kit that is specifically designed to
collect a forensic sample. The forensic blood collection kits (e.g. Tritech, Sirchie, Lynn Peavey)
use a 10-mL gray top collection tube manufactured by BD Vacutainer®
20
. Law enforcement
officers provide the kit to the phlebotomist or nurse on duty to obtain an evidentiary blood
sample.
Practitioner’s Note: Kits can be obtained at the manufacturer’s site and they are valuable to use
as exhibits and to cross-examine the person who collected the sample
21
. A typical kit should
include two gray top 10-mL tubes that have the proper amounts of sodium fluoride (100 mg) and
potassium oxalate (20 mg)
22
, a double ended needle (20 or 21 gauge), needle holder, a non-
alcohol disinfecting pad, a police officer’s report, a chain of custody document, use instructions
for the phlebotomist, use instructions for the police officer, a blood collection report, a consent
form, evidence seals for each tube, two evidence seals for the plastic storage container for the
tubes, two evidence seals for the cardboard box in which the sample is transported, a self closing
plastic bag to place the kit in for safety during transport, biohazard labels, and an absorbent pad
to be used when the needle is withdrawn from the draw site.
20
To obtain specific information concerning the vacutainer tube, access their web site: httr://www.bd.com/vacutainer
click on the product FAQ’s link on the left side, then scroll down to the section on common tube questions.
21
As example, the Lyn Peavey Blood-Alcohol collection kit, #05786, can be purchased for $6.95 and consists of the
following components:
Two gray top blood tubes containing 20 mg. potassium oxalate 100 mg. sodium fluoride
Needle and holder
Consent forms
Blood-collection report
Four blood-type labels for chain of custody
Providone-iodine prep pad
Four color-coded security seals
Absorbent materials
4-mil plastic Zip-Top Bag
Mailing carton
Instructions
22
The amount of sodium fluoride and potassium oxalate in each test tube must meet the preservative and
anticoagulant amounts that comply with the National Committee for Clinical Laboratory Standards standard. See
publication: Tubes and Additives for Venous Blood Specimen Collection; Approved Standard—Fifth Edition. NCCLS
document H1-A5 (ISBN 1-56238-519-4). NCCLS, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-
1898 USA, (2003).
25

Blood Collection Tube Guideline:
Color Top Additive Required Mixing Uses
Grey Potassium oxalate/Sodium fluoride 8 inversions Blood Alcohol
Yellow SPS or SPD 8 inversions Blood culture or DNA
Lavender Liquid K, EDTA 8 inversions Hematology
Red None None Serum Testing
Use of the Collection Tubes: The antiseptic pad/swab/towelette used to disinfect the draw site
and the package that it came in should be preserved after it is used so that it can be subsequently
tested to insure that it did not contain alcohol that could contaminate the sample.
A proper evidentiary blood draw should use the draw site antiseptic that is included in the
collection kit. Some medical facilities will use their own antiseptic to clean the draw site. This
break in procedure could lead to sample contamination. Medical facilities that conduct routine
venipunctures generally use a 70% isopropyl alcohol swab or towelette to disinfect draw sites.
Other disinfectants used by hospitals can contaminate the sample as well. Special instructions
are issued to not use isopropyl alcohol when collecting samples for blood alcohol
determinations.
Practitioner’s Note: The 70% isopropyl alcohol used in skin preparation for routine
venipuncture should not be used for blood alcohol determinations. Methanol can also affect
results. In addition, tincture of iodine contains alcohol and likewise should not be used to clean
the site. A non alcohol-containing alternative antiseptic such as chlorhexidine-gluconate or
regular soap and water should be used instead. Phlebotomy Essentials, 3
rd
edition, McCall and
Tankersley, page 373.
Since most medical facilities and/or phlebotomists do not draw forensic samples on a regular
basis, it is unlikely they will be familiar with the proper procedures to conduct a forensic draw.
It is very important to ascertain how the sample was drawn and what materials were used to
prepare the site. Inspection and/or analysis of the preserved towelette (proper procedures require
that the used towelette be preserved) will determine if the sample integrity has been
compromised.
Some agencies and medical facilities will use a benzalkonium chloride swab/ towelette (e.g.
manufactured by Triad) if it is determined that an individual may be allergic to iodine. The
problem with using this type of disinfectant is that it uses alcohol as a delivery medium. The
State of Colorado tested the Triad benzalkonium chloride towelette and discovered that there was
alcohol in the towelette and has subsequently ordered that this product not be used to collect
forensic blood samples because of the possible contamination
23
.
23
In a recent Motion to Suppress argued by the author before the Montgomery County District Court, the author
determined the cleaning agent ‘Hibiclens’ was used by the nurse on duty to sterilize the draw site.
26

DFS Comment: Headspace GC analysis is capable of separating and distinguishing ethanol
from isopropanol (isopropyl alcohol) and other alcohols. Accordingly, the contents of the
disinfection towelette are of no consequence unless it contains ethanol. In such case, the applied
ethanol could permeate the skin, enter the collected blood specimen and, therefore, represent a
true and significant contamination of the specimen. In the absence of ethanol as the cleansing
agent, no argument alleging “contamination” is scientifically justified. However, if another
method was used, such as a clinical analysis employing the enzyme alcohol dehydrogenase, it is
possible that the cross-reactivity of the enzyme for isopropanol occurred.
Protocol and Procedure: The person and/or agency (law enforcement agency or medical
facility) that collects the forensic sample should have specific and detailed written protocols for
the collection of the forensic sample.
Copies of the protocols should be obtained to determine if the collector was aware of and
followed the required protocols. These protocols can be obtained from the prosecution via a
formal disclosure request, or by issuing a subpoena duces tecum to the appropriate agency and/or
facility that drew the sample.
Be sure to check the qualifications of the person drawing the blood. In some cases, the person
drawing the blood is either not trained in blood drawing or not properly licensed. Even if
licensed and meeting the statutory requirements set forth in Code of Alabama, 32-5A-194, the
persons drawing the sample may have minimal training in this technical procedure. Physicians,
nurses and other licensed medical personnel may not be aware of the proper protocols for
obtaining an evidentiary sample.
Specific Protocol Required: All hospitals and other medical facilities should have specific
written protocols concerning the drawing of a forensic blood sample. Most of them require that
a forensic blood collection kit, provided by law enforcement, is used to collect the sample. The
instructions contained in the kit are to be followed precisely and only the contents of the kit are
to be used to collect the sample. Hospital supplies (antiseptic swabs/towelette, collection tubes,
needles, etc.) should not be used. The forensic kits are specifically designed and equipped to
facilitate a proper forensic sample collection; the use of non-authorized techniques or
components will invalidate the kit’s integrity and may invalidate the integrity of the sample.
DFS Comment: Blood is typically drawn pursuant to standard aseptic technique, which is to
insure both the subject and the specimen are not exposed to potential pathogens. This is typical
hospital policy and practice. The benefit of aseptic technique is safety to the subject and sterility
According to the manufacturer’s internet site, HIBICLENS Antiseptic/Antimicrobial Skin Cleanser is described as
the following: “HIBICLENS is an antiseptic antimicrobial skin cleanser possessing bactericidal activities.
HIBICLENS contains 4% w/v HIBITANE (chlorhexidine gluconate), a chemically unique hexamethylenebis
biguanide with inactive ingredients: Fragrance, isopropyl alcohol 4%, purified water, Red 40, and other ingredients,
in a mild, sudsing base adjusted to pH 5.0-6.5 for optimal activity and stability as well as compatibility with the
normal pH of the skin.” See, http://www.hibigeebies.com/sports/downloads/hibiclens_product_information.pdf
The fact that the skin cleaning agent used by the hospital to sterilize the draw site in this case contained 4% by
volume isopropyl alcohol may render subsequent analysis unreliable, unless the GC run was ethanol specific.
27
to the specimen. There is nothing unique about an evidentiary blood draw that an otherwise
competent phlebotomist must address beyond aseptic technique. Accordingly, a hospital may or
may not have a specific procedure for collecting forensic specimens as this is not the hospital’s
mission. What is different about an evidentiary blood draw, however, is not the draw itself but
the cleansing agent used to prior to the needle insertion; the labeling, sealing, custody and
delivery of the specimen to the forensic lab; and the type of forensic examination.
Preservation and Storage: The forensic kit should contain at least two gray stopped, 10-mL,
collection tubes. Gray stopped tubes will have sodium fluoride and potassium oxalate in pre-
determined quantity in the tube. Sodium fluoride will preserve glucose stability for up to 3 days.
If glycolysis is not prevented, the glucose concentration in a blood sample decreases at a rate of
l0 mg/dL per hour. Sodium fluoride also inhibits the growth of bacteria. The tubes should also
contain potassium oxalate, an anti-coagulant to prevent the blood from clotting. On occasion,
the wrong tubes are used and there is no, or an insufficient amount, of the anti-coagulant and
preservative in the sample. If these substances are not present, or are in insufficient quantities, or
are not properly mixed, neo-genesis or endogenous production of ethanol can occur.
Practitioner’s Note: Make sure the blood samples were collected in gray top tubes. Tubes with
other colored stoppers may not have the proper chemicals in them. Gray stopped collection
tubes contain a specific amount of sodium fluoride and potassium oxalate to prevent spoilage and
coagulation of the sample. Gray stopped tubes must be mixed immediately upon collection to
prevent clot formation fibrin generation. All BD Vacutainer tubes require immediate mixing
following collection. The procedures used to handle the blood sample after it has been collected
are another critical stage in maintaining sample integrity. If a sample is not properly mixed after
collection sample integrity will be compromised. BD Vacutainer states that each gray top tube
should be gently inverted eight to ten times to insure proper mixing of the anti coagulant and
preservative. Failure to follow the proper mixing protocol could cause the anti-coagulant and
preservative to not completely mix into the sample.
DFS Comment: Arguments over the type of collection tube involve two issues, namely the fluid
consistency of the blood and artifactual production of ethanol. Gray-top tubes are one of many
types of tubes used to collect specimens for clinical and chemical testing. These tubes are
preferred when collecting specimens for ethanol determinations because (1) the anticoagulant
preserves the fluid consistency of whole blood and (2) the preservative reduces the possibility of
production of ethanol through post-collection fermentation. Whereas these tubes are provided in
typical specimen collection kits, their use is not mandatory.
Collection of specimens in tubes lacking anticoagulant may give rise to a clotted specimen
which, when analyzed, may resemble serum more than whole fluid blood. While it has been
stated elsewhere that ethanol in whole blood rather than serum is the accepted basis for assessing
performance, it has also been stated that a result in serum may be reliably “converted” to one
which reflects whole blood. Furthermore, such conversion will reduce the reported result.
28
Accordingly, specimens collected in tubes lacking anticoagulant are acceptable for analysis and
the results may be interpreted with competent expert testimony.
Collection of specimens in tubes lacking preservative presents a greater opportunity for post-
collection microbiological decomposition. However, not all decomposition produces ethanol.
There is equal likelihood that ethanol will remain or diminish during decomposition.
Nonetheless, if decomposition does proceed with microorganisms capable of anaerobic
glycolysis (fermentation), then ethanol may be produced which would be otherwise
indistinguishable from what would otherwise be present from ingestion.
Decomposition is prevented in specimens primarily by reducing the presence of microorganisms
and secondarily by proper preservation and storage which is further discussed below. While
microorganisms are present in and on all humans, their numbers are limited in the blood of
healthy subjects. Anything otherwise would manifest as a systemic infection (septicemia)
requiring aggressive antibiotic treatment. Therefore, with sterile collection techniques, exposure
of the specimen to potentially decomposing microorganisms is minimized. Decomposition is not
uncommon in postmortem specimens because, unlike with living subjects, sterile environment
for collection may not exist. Accordingly, with specimens collected with sterile techniques from
living subjects, the likelihood of post-collection decomposition is minimal, if at all.
Analysis of blood samples: As stated previously, in most cases involving blood analysis, blood
samples are analyzed by the gas chromatograph process. The gas chromatograph process
essentially vaporizes a small portion of the sample and then that vapor is analyzed. This process
can also be used to identify the presence and concentration of the anticoagulant and preservative,
but most labs do not conduct these examinations unless specifically requested.
The internal standard (N-propyl alcohol) is added and mixed with the blood sample. Then, a
sample of this mixture is introduced into the gas chromatograph. Usually the amount introduced
is between one and ten micro-liters of solution, ideally three micro liters of solution. This is a
very small amount of chemical being tested. An eyedropper yields 50 micro-liters of liquid. The
laboratory will add chemicals to the sample that is being analyzed, (including N-propyl and
water as a standard), that are used to draw the alcohol (or salt out) from the sample and into the
vapor for analysis.
DFS Comment: Specimens are prepared for headspace GC analysis by diluting the specimen
11-fold with a solution containing saturated sodium fluoride. This equalizes the salt
concentrations among all specimens regardless of what may have existed in the undiluted
specimen, thereby minimizing potential variations in vaporization efficiencies.
No chemical test for anticoagulant is necessary in a forensic laboratory because its presence in a
specimen is indicated by the mere fact that the specimen is unclotted. If the specimen is clotted,
then anticoagulant was either absent or improperly mixed. Analysis of a clotted specimen should
29

be duly noted; whereupon the interpretation should consider the possibility that the specimen
was serum rather than whole blood.
Storage: If the blood sample is not properly stored, neo-genesis or endogenous production of
ethanol can occur in the blood sample. Simply put, the blood sample acts like a brewery and
ferments producing alcohol. This process occurs because blood is a living substance. It has
numerous micro organisms (yeasts, bacteria, etc.) naturally living within the blood. There is also
the possibility of contamination from exterior sources during the taking phase of the blood
collection process may have allowed yeasts and bacteria to enter the blood sample. During
storage inside the container vial, these micro organisms consume organic materials in the blood
(e.g. sugar) and produce alcohol as a waste product. This fermentation of blood may have an
impact upon the later reported ethanol concentration. This “neo-genesis” type of alcohol cannot
be distinguished from the alcohol suspected of being present in the driver or deceased, as
compared to alcohol generated from blood decay. Refrigeration will slow down, but not prevent,
the fermentation process and the production of alcohol
24
.
Practitioner’s Note: Gas chromatography analysis can only determine the amount of alcohol
present at the time of the analysis; it cannot tell the difference between alcohol that is present in
the sample due to ingestion by the sample’s donor or alcohol that is in the sample due to
endogenous production (fermentation) of alcohol or from some source of contamination.
Transportation of the Sample: After the sample is collected and packaged the officer will
transport the sample to an evidence holding area. During the time of transport the sample will be
exposed to room temperatures which are an optimum medium for bacterial growth (endogenous
alcohol production). The sample should be placed into an evidence refrigerator. Refrigeration
will slow these bacterial processes, but it will not stop them. The only way to stop this growth is
to freeze the sample and this is generally not done because it damages the blood cells. Failure to
analyze the sample within a time that is contemporaneous with the sample collection will
substantially increase to possibility of sample contamination by endogenously produced alcohol.
The longer the period of time between collection and analysis, the greater the possibility of
contamination or endogenous production
25
.
24
Studies have documented that temperatures are a critical factor in blood sample fermentation: J Forensic Sci.
1989 Jan; 34(1): 105-9. The effect of temperature on the formation of ethanol by Candida albicans in blood. Chang
J, Kollman SE. PharmChem Laboratories, Menlo Park, CA.
The effect of temperature on microbial fermentation in blood was studied. Specimens of human blood from a blood
bank were inoculated with Candida albicans, an organism capable of causing fermentation. A preservative was
added to a portion of the inoculated specimens. These inoculated specimens, as well as uninoculated blood, were
stored under various temperature conditions. Production of ethyl alcohol was monitored over a period of six
months. Fermentation was found to be highly temperature dependent, with refrigeration proving to be most
effective at inhibiting ethanol formation.
25
For a good example of how not to handle and transport a blood sample, see the case of Rafferty v. State, 799 So.
2d 243, 248 (Fla. App. 2001): No refrigeration of the blood sample for eight days. The blood sample in question was
taken under clinical conditions, then given to the first officer. The sample was taken to the local highway patrol
station by the first officer. Rather than being secured in a refrigerated container, the sample was placed in the
evidence room under unfrigerated conditions. The next day, a second officer took the blood sample from the
30

DFS Comment: Proper preservation and storage are necessary to insure that the condition and
composition of a biological specimen at the time of analysis reflect those at the time of
collection. Of particular concern is whether improper preservation and storage may lead to a
change in the ethanol content.
Preservation includes aseptic collection technique and chemical supplement (sodium fluoride).
While chemical preservation is preferred, the lack thereof does not necessarily result in
decomposition and artifactual ethanol production. The referenced study
(see footnote 24)
demonstrated that negligible ethanol was produced in unpreserved blood over 3 days at 37°C and
none was produced at room temperature over 10 days. Accordingly, a sterile yet unpreserved
specimen is stable for these periods.
Storage considerations are primarily controlled temperature. The preferred storage condition for
liquid blood is under refrigeration at 4°C, which is typical policy and practice in the laboratory.
Refrigeration reduces biological activity, including microbiological decomposition, and
evaporation of ethanol from the specimen. However, specimens stored not in the preferred
manner, such as during transport to the laboratory, do not necessarily become degraded in the
interim. The same referenced study
(see footnote 24) demonstrated that ethanol did not occur at
6°C, 22°C, and 37°C unless the specimens were inoculated with C. albicans. Such intentional
exposure is not encountered with specimens collected from healthy living subjects.
Degradation may also be physical (thermal). Thermal degradation typically manifests itself as a
coagulated or viscous specimen which may be more difficult to dispense for analysis. This may
influence the precision of the result, which is dependant upon the agreement of replicate
analyses. Specimen viscosity is apparent to the analyst and is duly noted. Exposure to heat far
in excess of room temperature may also promote evaporation of ethanol from the specimen
(especially where a tube contains only a small volume of specimen), which may give rise to a
lower result than would have otherwise been determined without such exposure. However,
thermal degradation is a physical-chemical phenomenon which does not produce ethanol.
There is no doubt that microbiological decomposition may proceed under the right conditions,
namely the right microbes, carbohydrates and temperature. Specimen decomposition manifests
itself as an atypical color or consistency which may progress from a brown-green color to black
sludge. There is also often a characteristic odor. These cues are apparent to the analyst and are
duly noted for consideration when results are interpreted. Decomposition also generates a host
of volatile, aromatic substances, some of which may appear in the chromatogram and may even
interfere or co-elute with the target (ethanol) or the internal standard (1-propanol). Accordingly,
microbiological decomposition sufficient to produce ethanol does not go unnoticed and
undocumented in the laboratory.
evidence room and drove the sample to the nearby crime lab that then prepared the sample for shipment via Airborne
Express to the testing laboratory. The sample was not reported as received at the forensic laboratory until six days
later. The Court’s opinion stated: “This case is a textbook example of how not to handle blood samples.”
31

OBTAINING THE CORRECT RESULT
WAS THE RESULT BASED ON WHOLE BLOOD OR SERUM?
Statutory limits in Alabama for ethanol in the blood are set at 0.02 g %, 0.04 g %, 0.05 g % and
0.08 g % in whole blood
26
. Therefore, one must consider what the whole blood ethanol
concentrations would have been even if another specimen was the basis for the analytical result.
The most common alternate specimen for ethanol analyses is serum, which is typically analyzed
in hospital and clinical laboratories. Because there is a predictable and measurable difference in
water content between serum and whole blood, a result in serum may be “converted” into an
equivalent whole blood result. Conversion requires a change of units from mg/dL to g/100 mL
and a reduction of 10% to 15% in concentration due to the lower water content of whole blood.
There are several ways to calculate this conversion. One is based upon the HCT of the subject,
the theory being the HCT is a reciprocal reflection of the water content of whole blood
27
.
Another method is based upon a comprehensive statistical examination of blood ethanol
determined with headspace GC versus concurrent serum ethanol concentrations determined with
clinical instrumentation
28
. The simplest method, however, is to reduce the serum ethanol content
by the relative difference determined for parallel analyses of serum and plasma, which is
generally held to be fifteen percent (15%)
29
. In this calculation, the serum ethanol result in the
units, mg/dL, is divided by 1150 to provide a whole blood ethanol equivalent in g/100 mL (g%).
It is also prudent to truncate the calculated result to two decimal places. This applies a bias
toward a lower calculated result. For example, a serum ethanol result of 139 mg/dL would
convert to 0.139 g/100 mL in serum, which is the equivalent of approximately 0.12 g/100 mL in
whole blood (139 mg/dL ÷ 1150 = 0.121 g/100 mL → 0.12 g/100 mL).
Clinical reports may include interpretational notes such as “legal limit” for a serum ethanol
concentration of 80 mg/dL because this result converts to 0.08 g/100 mL, a per se limit.
However, this notation is incorrect because only the units were converted and not the ethanol
concentration difference between serum and whole blood. A clinical ethanol result of 80 mg/dL
26
See, Code of Alabama, 1975, section 32-5A-191(a)(1): “A person shall not drive or be in actual physical control of
any vehicle while: (1)There is 0.08 percent or more by weight of alcohol in his or her blood.”
Code of Alabama, 1975, section 32-5A-191(b): “A person who is under 21 years shall not drive …if there is .02
percentage or more by weight of alcohol in his or her blood.”
Code of Alabama, 1975, section 32-5A-191(c)(1): “A school bus or day care driver shall not drive or be in actual
physical control …if there is greater than .02 percentage by weight of alcohol in his or her blood.”
Code of Alabama, 1975, section 32-5A-191 (c)(2): “A person shall not drive or be in actual physical control of a
commercial motor vehicle …if there is .04 percentage or greater by weight of alcohol in his or her blood.”
27
Shajani, et al., Can. Soc. Forens. Sci. J. 22(4):317-320 (1989)
28
Barnhill, et al., J. Anal. Toxicol. 31(1):23-30 (2007)
29
Charlebois, et al., J. Anal. Toxicol. 20(1):171-178 (1996)
32
is actually the equivalent of a whole blood ethanol result of approximately 0.07 g/100 mL. For a
whole blood result to be 0.08 g/100 mL, a serum ethanol result could be as high as 102 mg/dL
(102 mg/dL ÷ 1150 = 0.089 g/100 mL → 0.08 g/100 mL). Reliance upon the serum result
without a correct and complete conversion could lead to a misinterpretation of the result.
Whole Blood or Serum? A critical question that must be asked concerning the sample that was
analyzed: Was the sample whole blood or serum or plasma? This question is of particular
importance if the blood alcohol determination was performed in a hospital lab.
Whole blood is composed of cellular material, plasma and fibrinogen (clotting agent). Hospitals
test serum or plasma (whole blood is centrifuged or filtered to remove the cellular material and
fibrinogen). Medical blood draws are primarily concerned as to whether alcohol is present and
not concerned about the specific amount or concentration of the alcohol. A forensic or
evidentiary blood draw is concerned with precise concentration of alcohol in the sample.
Testing plasma or serum is less cumbersome than testing whole blood, but there are problems
associated with plasma (e.g. when you centrifuge the blood sample, you take the solid, cellular
material out, but you leave the same amount of alcohol in a smaller volume of liquid). This
process artificially increases the concentration of alcohol in the liquid-which can lead to
erroneously high test result. Hospital labs test serum or plasma but report it as “blood alcohol.”
However, whole blood ethanol is not the same as serum ethanol or plasma ethanol.
Serum is plasma with the fibrinogen (clotting material) removed. Serum is collected when the
blood sample is allowed to actually clot. When the blood clots, a clear liquid (serum) forms over
the blood. While serum and plasma alcohol concentrations are not significantly different, both of
these tests produce results that are very different from the results produced by the analysis of
whole blood. When plasma or serum analysis is used, the blood alcohol content will appear on
average 14 % to 16 % higher than whole blood.
In a study of the difference between whole blood ethanol and serum ethanol and plasma ethanol
reported in the Journal of Analytical Toxicology, Vol. 11, November/December 1987,
“Comparison of Plasma, Serum, and Whole Blood Ethanol Concentrations” by forensic
scientists Charles L. Winek and Mark Carfagna, fifty subjects who had consumed alcohol were
tested by taking a whole blood sample and simultaneously taking a second sample for blood
serum. The average relative difference in reported ethanol concentration between whole blood
and serum or plasma was 1.14 ± 0.02 across all fifty subjects. In other words, the plasma-serum
series of tests showed a higher ethanol result by 14% as compared to the whole blood tests, with
both samples measured by direct injection gas chromatography.
As Winek and Carfagna noted: “A person with an ethanol concentration of 92 mg/dL in whole
blood could have a reported concentration above 100 mg/dL if either serum or plasma is
analyzed.” In other words, according to Winek and Carfagna, a reported hospital lab result of
0.10 % BAC [mg/dL] using blood serum would be actually 0.092 % BAC if whole blood was
analyzed. In view of the significant penalties for a conviction of alcohol related traffic collision,
33
it is imperative that competent counsel understand which method — whole blood or blood
serum/blood plasma- was used to determine the subject’s reported blood alcohol level.
A second scientific treatise, Clinical Chemistry, Volume 39, No. 11, 1993, confirmed the earlier
Winek and Carfagna study by running tests of blood samples taken from 211 persons. Professor
Petrie M. Rainey of the Department of Laboratory Medicine, Yale University School of
Medicine, using the gas chromatography method of analysis at the Yale University School of
Medicine laboratory facility, determined the median ratio to be 1.15. The common range of
deviation among the 200+ individuals examined was 1.14 to 1.17.
The report noted to a 95% degree of confidence, the range was 1.14 to 1.17, and the median ratio
of all subjects was 1.15. As Professor Rainey noted:
“Clinical laboratories have traditionally measured ethanol concentrations in serum
or plasma. All state laws that define driving while intoxicated are written in terms
of whole-blood concentrations. Because treatment of injuries takes precedence
over collection of evidence, alcohol concentrations obtained in the emergency
department are often the only measurements available on injured motorists.
These measurements may be used as legal evidence in both civil and criminal
proceedings. However, differences between serum and whole-blood alcohol
concentrations have created difficulty in interpreting serum concentrations under
legal statutes.”
Professor Rainey’s study determined, on average, the correct method to convert serum or plasma
into the grams per cent by weight calculation was to divide by 1.15. “The median whole-blood
alcohol concentration can be calculated by dividing the serum alcohol by 1.15 for a result in
mg/dL or by 1150 for a result in weight percent.” However, this computation is not precise for
all persons, and there is a degree of variance in the concentration of ethanol between whole
blood and blood plasma. Clinical Chemistry, Vol. 39, No. 11 at pages 2288-2292.
For Additional Information:
There are two publications that are recommended reference material in understanding alcohol-
related evidence in civil and criminal trials and legal proceedings. Both publications are
recommended for law enforcement, defense attorneys, and prosecutors:
1) Garriott’s Medicolegal Aspects of Alcohol, 5
th
Edition (2008)
by James C. Garriott, Ph.D, et. al.
Available from: Lawyers and Judges Publishing Company, P.O. Box 30040, Tucson, Az. 85751
(800) 209-7109
2) Attacking and Defending Drunk Driving Tests (Rev. 2 3/10)
by Donald J. Bartell, Mary Catherine McMurry, and Anne D. ImObersteg
Available from: James Publishing, Inc., 3505 Cadillac Ave., Suite H, Costa Mesa, CA. 92626
(800) 440-4780
34

ABOUT THE AUTHORS
Patrick Mahaney is a criminal defense attorney in Montgomery, Alabama, concentrating his
practice in defense of DUI and traffic offense, drug violations, and driver license cases. He also
serves as local counsel to the Alabama Police Benevolent Association and represents P.B.A.
members in employment law issues.
Mr. Mahaney served twenty-two years as a state trooper with the Alabama Department of Public
Safety (1978-2000), including duty in uniform patrol, headquarters staff, and as assistant legal
counsel for the Department. After retirement from state service, Mr. Mahaney served overseas
with the U.S. State Department’s civilian police programs in Kosovo, Jordan, and Iraq where he
supervised police reform and development efforts. He served 18 months in Iraq as the executive
officer for the U.S. Department of State’s civilian police mission.
Mr. Mahaney received his B.A. from The Citadel, Charleston, S.C. and his law degree from
Jones School of Law in Montgomery, Alabama. He was admitted to the Alabama state bar in
1989 and is member of the bar of the state of Alabama and the federal courts, to include the U.S.
Supreme Court.
Law Office of Patrick Mahaney
8244 Old Federal Road
Montgomery, Alabama 36117
(334) 277-3974
patrick@mahaneylaw.com
Jack R. Kalin, PhD, DFTCB is a forensic toxicologist in Birmingham, AL where he is the
current Toxicology Discipline Chief for the Alabama Department of Forensic Sciences. Dr.
Kalin received a BSc in biochemistry from McGill University (Montreal, PQ, 1972) and a PhD
in biochemistry and molecular biology from the University of Florida (1979). He previously
conducted research with experimental drugs at Southern Research Institute in Birmingham (9
years, 19 publications) and worked as a forensic toxicologist with the Broward County (Florida)
Medical Examiner. Dr. Kalin has been with the Department of Forensic Sciences for 19 years (4
publications, numerous presentations) and is certified in forensic toxicology by the Forensic
Toxicologist Certification Board, of which he is a current board member and past president. Dr.
Kalin belongs to numerous forensic professional organizations and is a Fellow of the American
Academy of Forensic Sciences. Dr. Kalin is a lecturer in the Justice Sciences program at the
University of Alabama at Birmingham and at Jefferson State University.
Jack R. Kalin, PhD, DFTCB
Alabama Department of Forensic Sciences
2026 Valleydale Road
Hoover, AL 35244-2095
205-982-9292
35
TECHNICAL REVIEWER
Jimmie L. Valentine, B.S., B.S., M.S., Ph.D.
Dr. Valentine holds B.S. degrees in both Biology and Chemistry from Centenary College of
Louisiana and received his M.S. and Ph.D. degrees from The University of Mississippi in
Medicinal Chemistry. In 2008, Dr. Valentine retired from the University Of Arkansas College Of
Medicine where he served for 19-years as Professor of Pediatrics, Pharmacology, and Myeloma
Research.
Prior to Dr. Valentine’s tenure at the University of Arkansas, Dr. Valentine was a faculty member
at the University of Missouri-Kansas City School of Medicine (1973-1978) and Oral Roberts
University School of Medicine in Tulsa, Oklahoma (1978-1990) where he served as Professor
and Chairman of Pharmacology from 1984-1990. He was recognized as the Outstanding Faculty
Member, an award bestowed by the medical students in 1984.
At all the academic institutions where Dr. Valentine served as a faculty member he also directed
certified toxicology laboratories and had active research programs in the fields of drugs of abuse,
performing and developing analytical techniques for determining drugs of abuse, and correlating
levels found in humans with behavioral effects. Dr. Valentine has published 60 papers in the
scientific and medical literature and has written 12 chapters in professional treatises and is co-
author of 3 books. Dr. Valentine has presented numerous training seminars on the correlation of
drug use and behavioral effects in relationship to levels determined in physiological fluids both
in living and post-mortem situations. Dr. Valentine is Board certified in applied clinical
pharmacology and as a forensic examiner. He is also a Certified Flight Instructor and active
pilot. Dr. Valentine has been certified as an expert witness in numerous civil and criminal trials
and administrative hearings concerning drug findings. Dr. Valentine now serves as a consultant
in medical pharmacology and toxicology.
Dr. Jimmie L. Valentine
Medical Pharmacology and Toxicology Consulting
P.O. Box 1487 P.O. Box 924
Ocean Springs, MS 39564 Americus, GA 31709
(501) 258-9242
36
