
02/07/2020 Page 1 of 73
VACCINES FOR CHILDREN
PROGRAM MANUAL
FOR ILLINOIS VFC PROVIDERS
February 7, 2020

02/07/2020 Page 2 of 73
CONTENTS
1. Overview of the VFC Program ..................................................................................................... 5
Vaccines for Children (VFC)................................................................................................................... 5
Advisory Committee On Immunization Practices (ACIP) ...................................................................... 5
VFC and I-CARE in Illinois ...................................................................................................................... 6
Fee Caps on Vaccine Administration .................................................................................................... 6
2. Provider Enrollment ................................................................................................................... 7
VFC Program Requirements Summary ................................................................................................. 7
Recertification of Annual Enrollment ................................................................................................... 9
VFC Enrollment Visits ............................................................................................................................ 9
Education Requirement ...................................................................................................................... 10
Memorandum of Understanding (MOU) with a FQHC or RHC ........................................................... 10
Termination of Enrollment Agreement .............................................................................................. 10
3. Eligibility .................................................................................................................................. 12
VFC Eligibility Criteria .......................................................................................................................... 12
American Indian or Alaska Native (AI/AN) .......................................................................................... 12
VFC Eligibility and Insurance Situations .............................................................................................. 13
Insured Children with Medicaid Title XIX (19) as Secondary Insurance ............................................. 14
Medicaid as Secondary Insurance and High-Deductible Insurance Plans .......................................... 15
Underinsured ...................................................................................................................................... 15
Health Care Sharing Ministries ........................................................................................................... 15
VFC Eligibility in Special Circumstances .............................................................................................. 16
State of Residency .............................................................................................................................. 16
Provider Responsibility to Screen for VFC Eligibility ........................................................................... 16
VFC Eligibility Decision Tree and Scenario Chart ................................................................................ 16
4. Children’s Health Insurance Program (CHIP) .............................................................................. 18
5. Vaccine Staff and Training ........................................................................................................ 21
Vaccine Coordinators .......................................................................................................................... 21
Staff Training ....................................................................................................................................... 22
6. Vaccine Storage and Temperature Monitoring Equipment ......................................................... 23
Vaccine Cold Chain .............................................................................................................................. 23
Refrigerator and Freezer Units ........................................................................................................... 24
Equipment Types ................................................................................................................................ 24
Purpose-Built Vaccine Storage Units .................................................................................................. 25
02/07/2020 Page 3 of 73
Storage Unit Placement ...................................................................................................................... 26
Storage Unit Doors .............................................................................................................................. 26
Stabilizing Temperatures in New, Moved, and Repaired Units .......................................................... 26
Temperature Ranges ........................................................................................................................... 27
Digital Data Loggers ............................................................................................................................ 27
Power Supply ...................................................................................................................................... 28
Vaccine Unit Setup .............................................................................................................................. 29
7. Mobile Vaccine Clinics .............................................................................................................. 30
8. Off-Site Vaccine Clinics ............................................................................................................. 31
9. Ordering and Receiving Vaccines............................................................................................... 33
Placing Vaccine Orders ....................................................................................................................... 33
Patient Population Profiles ................................................................................................................. 34
Tracking Vaccine Orders ..................................................................................................................... 34
Borrowing Vaccines ............................................................................................................................ 34
Receiving and Unpacking Vaccine Shipments .................................................................................... 35
Merck Frozen Shipments .................................................................................................................... 36
Identifying the Vaccines by Funding Type .......................................................................................... 36
Identifying the Split Doses .................................................................................................................. 38
10. Inventory Management ............................................................................................................ 40
Storing Vaccines .................................................................................................................................. 40
Vaccine Storage with Only One Fund Type in a Box ........................................................................... 40
Vaccine Storage with More Than One Fund Type in a Box ................................................................. 40
Vaccine Management ......................................................................................................................... 41
Daily Tasks ........................................................................................................................................... 41
Weekly Tasks ....................................................................................................................................... 41
Monthly Tasks (Or More Often As Needed) ....................................................................................... 41
Annual Tasks (Or More Often As Needed) .......................................................................................... 41
Routine Maintenance ......................................................................................................................... 42
Best Practices ...................................................................................................................................... 42
Temperature Excursions ..................................................................................................................... 43
Freezer Defrost Cycles and Temperature Excursions ......................................................................... 43
Provider-to-Provider Transfer of Vaccines ......................................................................................... 44
Transport or Shipping ......................................................................................................................... 44
Transfer Procedure ............................................................................................................................. 45
Vaccine Transportation Guidelines ..................................................................................................... 46
02/07/2020 Page 4 of 73
Transport System Recommendations ................................................................................................. 49
Moving to a New Location .................................................................................................................. 49
Expired, Spoiled, or Wasted Vaccines ................................................................................................. 50
Return Mailing Labels ......................................................................................................................... 52
Multidose vials .................................................................................................................................... 52
11. Vaccine Management Plan........................................................................................................ 56
Standard Operating Procedures ......................................................................................................... 56
Emergency Response .......................................................................................................................... 57
12. VFC Site Visits ........................................................................................................................... 58
VFC Compliance Visit .......................................................................................................................... 58
Storage and Handling Site Visit ........................................................................................................... 59
Conducting the Site Visit ..................................................................................................................... 59
Following Up After the Site Visit ......................................................................................................... 59
13. Vaccine Loss and Replacement.................................................................................................. 60
Definitions ........................................................................................................................................... 60
Situations Requiring Vaccine Replacement ........................................................................................ 60
Expired Vaccine ................................................................................................................................... 60
Spoiled Vaccine ................................................................................................................................... 60
Situations Not Requiring Vaccine Replacement ................................................................................. 62
Procedures For Vaccine Replacement ................................................................................................ 62
Additional Information ....................................................................................................................... 62
Procedure to Appeal a Vaccine Replacement .................................................................................... 63
14. Fraud and Abuse ...................................................................................................................... 64
Overview ............................................................................................................................................. 64
Fraud and Abuse Policy ....................................................................................................................... 65
Examples of Fraud and Abuse ............................................................................................................. 65
Allegations of Suspected Fraud and Abuse ........................................................................................ 66
Fraud and Abuse Contacts .................................................................................................................. 67
Ongoing Provider Monitoring Procedures .......................................................................................... 67
Reporting VFC Provider Terminations ................................................................................................ 67
Appendices ...................................................................................................................................... 68
VFC Eligibility Status Codes ................................................................................................................. 69
VFC Tip Sheets..................................................................................................................................... 70
Glossary of Important VFC Terms ....................................................................................................... 71

02/07/2020 Page 5 of 73
1. OVERVIEW OF THE VFC PROGRAM
VACCINES FOR CHILDREN (VFC)
The Vaccines for Children (VFC) program is a federally-funded program from the Centers for Disease
Control and Prevention (CDC) that provides vaccines at no cost to children who might not otherwise be
vaccinated because of an inability to pay. The benefits of the VFC program include:
• Reducing referrals of children from private providers to state health departments for
vaccination.
• Saving VFC-enrolled providers out-of-pocket expenses for vaccine.
• Eliminating or reducing vaccine cost as a barrier to immunizing eligible children.
VFC providers contribute to increased immunization coverage level rates and reduced delays in
immunizations and, subsequently, the risk of serious illness or death from vaccine-preventable diseases.
The Illinois Department of Public Health (the Department) administers the VFC program to provide
immunizations for children through the age of 18 who are uninsured (“self-pay”), Medicaid Title XIX
(19)-eligible, American Indian or Alaskan Native. Underinsured children (children who have limited
coverage or caps on the amount of vaccines allowed annually) can access VFC vaccines recommended by
the CDC’s Advisory Committee on Immunization Practices (ACIP) at participating federally qualified
health centers (FQHC) and rural health clinics (RHC), or local health departments (LHD) under an
approved deputization agreement. All VFC providers must offer all ACIP-recommended vaccines for the
populations they serve.
Children with Title XXI (21) or State-funded coverage (as shown in the Illinois Department of Healthcare
and Family Services MEDI system in the “Special Information” section) have Children’s Health Insurance
Program (CHIP) coverage are not eligible for VFC vaccines and must receive CHIP vaccines. As of
September 1, 2019, CHIP vaccines will be provided through the VFC program.
This program manual is intended for providers currently enrolled in the Illinois VFC program. Providers
located within the City of Chicago should contact the Chicago Department of Public Health via e-mail at
.
The CDC Vaccine Storage and Handling Toolkit provides guidance and best practices for all health care
providers (including VFC-enrolled providers) and is the source for information sited in this manual. The
CDC Vaccine Storage and Handling Toolkit is available at
http://www.cdc.gov/vaccines/recs/storage/toolkit/storage-handling-toolkit.pdf
.
ADVISORY COMMITTEE ON IMMUNIZATION PRACTICES (ACIP)
The Advisory Committee on Immunization Practices (ACIP) is a federal advisory committee that was
established in 1964 to provide advice and guidance on the most effective means to prevent vaccine-
preventable diseases. In 1993, Congress gave the ACIP unique legal authority to determine
recommendations for the routine administration of vaccines to children and adults in the civilian
population. The ACIP is the only entity in the federal government that makes such recommendations.
These recommendations include:
• Age for vaccine administration
• Number of doses and dosing interval
• Precautions and contraindications

02/07/2020 Page 6 of 73
Major functions of the ACIP are as follows:
• Develops technical recommendations on vaccine use and immunization practices.
• Approves vaccines to be provided through the VFC program.
• Recommends immunization schedules that are harmonized with recommendations of other
advisory groups, such as the American Academy of Pediatrics (AAP) and the American Academy
of Family Physicians (AAFP).
VFC AND I-CARE IN ILLINOIS
The Illinois Immunization Section requires VFC providers to be enrolled and active users of the Illinois
Comprehensive Automated Immunization Registry Exchange (I-CARE). Additional information and forms
for I-CARE are available at
http://www.dph.illinois.gov/topics-services/prevention-
wellness/immunization/icare. The Immunization Section has integrated its VFC enrollment and vaccine
management functions into I-CARE. This integration allows for greater accountability and programmatic
oversight.
All Illinois VFC providers must provide individual patient immunization records on how each VFC vaccine
was administered. The individual patient immunization records can either be manually entered directly
into I-CARE or can be electronically transmitted to I-CARE from the provider's electronic medical record
(EMR) system. VFC providers not in compliance will not be able to continue participating in the VFC
program.
FEE CAPS ON VACCINE ADMINISTRATION
Illinois VFC providers may charge a vaccine administration fee for non-Medicaid VFC-eligible children
only. Providers are not allowed to bill VFC-eligible children for the cost of the VFC vaccine. As of
January 1, 2013, the vaccine administration fee may not exceed the administration fee cap of $23.87 per
vaccine dose. VFC providers may not deny administration of a publicly purchased vaccine to an
established patient because the child's parent/guardian/individual of record is unable to pay the
administration fee.
Effective January 1, 2020, VFC providers may issue a single bill for the administration fee for non-
Medicaid VFC-eligible children within 90 days of vaccine administration.
Unpaid VFC vaccine administration fees may not be sent to collections and VFC providers may not refuse
to vaccinate an eligible child whose parents have unpaid vaccine administration fees.
02/07/2020 Page 7 of 73
2. PROVIDER ENROLLMENT
All VFC providers must complete the enrollment annually to recertify their participation in the VFC
program. Annual enrollment for the VFC program is submitted through I-CARE and supporting
documentation faxed or emailed to the Department.
Providers who are new to the VFC program will need to complete the I-CARE application first.
Information and forms for enrollment in I-CARE are available at
http://www.dph.illinois.gov/topics-
services/prevention-wellness/immunization/icare. Providers may contact the I-CARE team at
[email protected] to check the status of an I-CARE enrollment application.
VFC PROGRAM REQUIREMENTS SUMMARY
REQUIREMENT
COMPONENT
VFC Provider
Requirements
VFC providers must:
• Be licensed in Illinois to administer vaccines to children aged 18 and younger.
• Be willing and able to follow all VFC program requirements, policies, and
procedures, including participation in site visits and educational opportunities.
• Have the capacity to order, receive, manage, store, and monitor the
temperature of public vaccines.
• Be open at least 4 consecutive hours for three days a week to receive VFC
vaccines.
Provider
Agreement
• Providers must complete and sign CDC’s Provider Agreement.
• The medical director in a group practice must be authorized to administer
pediatric vaccines under state law.
• The provider signing the Provider Agreement on behalf of a multi-provider
practice must have authority to sign on behalf of the entity.
• All licensed health care providers in an enrolled practice and their
corresponding professional license numbers must be listed in the VFC
Enrollment Form.
• Providers must submit a Provider Population Profile at initial program
enrollment and updated at least annually or when order patterns indicate a
change.
Patient Eligibility
Screening
• Providers must screen and document patient eligibility screening in the
patient’s permanent medical record (paper-based or electronic medical record)
using the VFC Patient Eligibility Screening Record or document the required
elements in the electronic medical record.
Vaccine
Management
VFC providers must comply with vaccine management guidelines in the CDC’s
Vaccine Storage and Handling Toolkit, including:
• Correct storage units;
• Digital data loggers (DDLs) with continuous monitoring capabilities and a
current Certificate of Calibration;
• Receiving and documenting vaccines;
• Daily monitoring and recording of unit temperatures, including responding to
any temperature excursion;
• Managing expired, spoiled, or wasted vaccine;
• Vaccine handling and preparation; and
• Procedures for emergency situations.

02/07/2020 Page 8 of 73
REQUIREMENT
COMPONENT
Vaccine
Management
Plan
VFC providers must have standard operating procedures for routine and emergency
vaccine management:
• Contact information for current primary and backup vaccine coordinators;
• Provider staff roles and responsibilities;
• Documented training related to vaccine management;
• Proper storage and handling practices, including how to handle a temperature
excursion;
• Procedures for vaccine ordering, receiving, inventory control, stock rotation,
and handling vaccine loss and waste;
• Procedures for emergency situations, including transport, equipment
malfunction, power failure, and natural disaster; and
• Plans must be updated annually or more frequently as needed.
Immunization
Schedule
VFC providers must comply with:
• Current ACIP recommendations and VFC resolutions;
• Making available the vaccines identified in the Provider Profile based on the
provider type and population served, including non-routine vaccines, if
applicable;
• Understanding state laws related to vaccination requirements and acceptable
vaccine exemptions; and
• Using ACIP recommendations and vaccine package inserts to understand
contraindications for each vaccine type available through the VFC program.
National
Childhood
Vaccine Injury
Act (NCVIA)
VFC providers must comply with:
• Obtaining and distributing the most current vaccine information statements for
all vaccines included in the National Vaccine Injury Compensation Program;
• Following the record-keeping requirements for the NCVIA; and
• Reporting adverse reactions to VAERS.
Fraud and Abuse
• VFC providers must operate in a manner intended to avoid fraud and abuse.
Vaccine
Restitution
• VFC providers agree to replace vaccines purchased with state and federal funds
that are deemed non-viable due to provider negligence on a dose-for-dose basis
with privately purchased vaccines.
VFC Visits
• VFC providers agree to VFC program site visits, which may include compliance
visits, unannounced storage and handling visits, or educational site visits.

02/07/2020 Page 9 of 73
RECERTIFICATION OF ANNUAL ENROLLMENT
All VFC providers are required to submit an annual enrollment to recertify their participation in the VFC
program. Enrollment documentation is available in and submitted through I-CARE with supporting
documentation faxed or emailed to the Department.
Providers will need to read and agree to the following policies, which are available in I-CARE and
updated annually:
• VFC Enrollment Agreement Terms
• VFC Provider Enrollment Policy
• VFC Loss and Replacement Policy
Provider agreement forms must be signed annually by the medical director or the equivalent in a group
practice. The health care provider signing the agreement must be a practitioner authorized to
administer pediatric vaccines under state law. The practitioner will also be held accountable for
compliance by the entire organization and its VFC providers with the responsible conditions outlined in
the Provider Enrollment Agreement.
All licensed health care providers in the enrolled practice – and their corresponding professional license
numbers – must be listed on the provider agreement form.
According to Section 1928 (c) (1) (A) of the Social Security Act (42 U.S.C. 1396s (c) (1) (A) the following
providers qualify to be VFC program-registered providers:
Health care providers “licensed or otherwise authorized for administration of pediatric vaccines
under the law of the State in which the administration occurs” (subject to section 333 (e) of the
Public Health Service Act, which authorizes members of the Commissioned Corps to practice).
The CDC Provider Agreement form represents the provider’s agreement to comply with all the
conditions of the VFC program, as well as ensuring that the practice/clinic/facility and all providers listed
on the agreement will adhere to the requirements of the program.
Providers re-enrolling after an absence must complete the annual enrollment. Re-enrolling providers
may be required to resolve any inventory issues or outstanding vaccine replacements before a new
enrollment may be approved.
VFC ENROLLMENT VISITS
All providers newly enrolling or re-enrolling after an absence in the VFC program must have an
enrollment site visit before being approved to order VFC vaccines. The purpose of this visit is to:
• Educate providers about VFC program requirements.
• Educate providers on proper vaccine storage and handling.
• Certify providers have the appropriate resources to implement requirements.
• Confirm providers know whom to contact if problems arise, especially with storage and handling
issues.
• Complete a Vaccine Management Plan.
A VFC storage and handling visit may be conducted approximately within three to six months after the
enrollment visit and a compliance site visit within six to 12 months after the enrollment visit.

02/07/2020 Page 10 of 73
By the end of the enrollment visit, the provider and staff will understand:
• The eligibility requirements for the VFC program.
• Where to refer underinsured children for VFC vaccine if the child is not eligible in that practice –
federally qualified health center (FQHC), rural health clinic (RHC) or a deputized local health
department (LHD).
• How and when to screen and document VFC eligibility appropriately.
• How to screen and document VFC eligibility in special populations.
• How to identify CHIP-covered patients and the vaccine stock for use with CHIP-covered patients.
EDUCATION REQUIREMENT
All VFC vaccine coordinators are required to complete annual VFC education on vaccine storage and
handling. Documentation of training must be retained and submitted with annual enrollment, as well as
reviewed during site visits. Education is available through VFC compliance site visits, VFC educational
visits, or through the CDC online training, “You Call The Shots – Module 10 – Storage and Handling,”
available at https://www.cdc.gov/vaccines/ed/youcalltheshots.html
. Trainings offered by other states
or projects (such as the Chicago VFC program or the Pink Book trainings) do NOT meet the Illinois VFC
training requirement. A VFC training log is available in the Vaccine Management Plan for providers to
document training received. Copies of training certificates must be attached to the training log.
MEMORANDUM OF UNDERSTANDING (MOU) WITH A FQHC OR RHC
LHDs who wish to qualify to vaccinate underinsured children using VFC vaccine must be established and
recognized as a FQHC, RHC or an agency with FQHC delegate authority. A FQHC with a Health
Resources and Services Administration PHS Section 330 grant award notice or an RHC with a
Department RHC status letter must use the CDC’s memorandum of understanding (MOU) request to
delegate authority to vaccinate underinsured children on their behalf. Providers should retain a copy of
their MOU and submit it annually during VFC enrollment recertification to continue to be able to
administer VFC vaccine to underinsured patients. Completed MOUs will be reviewed annually and
updated as needed. For more information on deputization agreements, please contact the VFC program
.
TERMINATION OF ENROLLMENT AGREEMENT
The Illinois VFC program or the provider may terminate this agreement at any time or if there is failure
to comply with these requirements. If the agreement is terminated, the provider agrees to properly
return any unused VFC vaccines within 30 days of the termination date. VFC vaccines may not be used
after the unenrollment or termination date.
Unfortunately, some circumstances may occur that necessitate VFC providers unenrolling from their role
as an approved provider. The cause for these circumstances may vary, but timely and appropriate
notification by the provider is desired and expected. The following steps should occur:
• The clinic should complete the VFC provider unenrollment form available in I-CARE and fax or e-
mail to the Illinois VFC program. Be sure to include the handwritten temperature logs for the
previous three months and the current physical VFC inventory you have in stock.
• If the enrollment agreement is terminated, the provider agrees to properly return any unused
VFC vaccine within 30 days of the termination date. The provider may not continue to
administer VFC vaccines after the termination date.
02/07/2020 Page 11 of 73
• If the clinic can provide documentation of the cold chain being maintained, the clinic must find
another VFC provider to transfer their remaining vaccines. The Illinois VFC program will review
documentation of the cold chain and advise the provider of next steps.
• The Illinois VFC program will contact the provider to follow up on the unenrollment notification.

02/07/2020 Page 12 of 73
3. ELIGIBILITY
VFC ELIGIBILITY CRITERIA
Providers must screen, document, and verify VFC eligibility with every immunization visit before
administering vaccines. Providers must check the eligibility status in the MEDI system
(http://www.illinois.gov/hfs/MedicalProviders/EDI/medi/Pages/default.aspx
) or an equivalent system
receiving the HFS 270/271 electronic transaction data.
To be eligible to receive VFC vaccine, children (regardless of their state of residency) through the age of
18 (until the day of their 19
th
birthday) must meet at least one of the following criteria:
VFC ELIGIBILITY CRITERIA
DEFINITION
American Indian or
Alaska Native (AI/AN)
This population is defined by the Indian Health Care Improvement Act (25
U.S.C. 1603). (AI/AN children are VFC-eligible under any circumstance.)
Medicaid-eligible
Children who are eligible for the Medicaid program Title XIX (19). For the
purposes of the VFC program, the terms “Medicaid-eligible” and “Medicaid-
enrolled” are used interchangeably.
Uninsured
Children not covered by any health insurance plan
Underinsured
Underinsured means the child has health insurance, but the insurance
policy:
• Does not include any vaccines;
• Does not include all vaccines recommended by the Advisory Committee
on Immunization Practices (ACIP); or
• Has a fixed dollar limit or cap for vaccines.
Underinsured children are only eligible to receive VFC vaccines at a FQHC,
RHC, or a deputized provider.
Any patient 19 years of age or older is NOT eligible for VFC vaccines, regardless of insurance status.
Occasionally, children may be VFC-eligible for more than one eligibility category. A provider must select
and document the VFC eligibility category that will require the least amount of out-of-pocket expenses
to the parent/guardian for the child to receive necessary immunizations. VFC is an entitlement
program and participation in VFC is not mandatory for an eligible child.
Children with Title XXI (21) or State-funded coverage have CHIP coverage are not eligible for VFC
vaccines and must receive CHIP vaccines. See section 4 for information on vaccines for children with
CHIP coverage.
AMERICAN INDIAN OR ALASKA NATIVE (AI/AN)
The American Indian or Alaska Native (AI/AN) population, for the purposes of the VFC program, is
defined by the Indian Health Care Improvement Act [25 U.S.C. 1603]. AI/AN children are VFC-eligible
under any circumstance. However, because VFC is an entitlement program, participation is voluntary.
When an AI/AN child also fits a second VFC eligibility category, the provider should always choose the
category that will cost less for the family. Depending on the facility where an AI/AN parent chooses to
have their child vaccinated, the parent may be responsible for the vaccine administration fee if the
vaccines are delivered through the VFC program. Therefore, if the child has private insurance (non-
grandfathered plan under the Affordable Care Act (ACA) of 2010) or is enrolled in the CHIP program, it

02/07/2020 Page 13 of 73
may result in fewer out-of-pocket costs for the child to receive vaccinations through these programs
than through VFC, as there would be no cost-sharing. Likewise, if the AI/AN child is also Medicaid-
eligible, Medicaid should be used for the administration fee because it will provide the least out-of-
pocket expense.
VFC ELIGIBILITY AND INSURANCE SITUATIONS
Child’s Insurance Status
VFC-Eligible?
VFC Eligibility Category
Enrolled in Medicaid Title XIX (19)
Yes
Medicaid (V02)
Has private health insurance plan with
Medicaid Title XIX (19) as secondary
insurance
Yes
Medicaid (V02)
Has health insurance covering all
vaccines, but has not yet met plan’s
deductible or paid for other services
received at visit
No
Insured (V01). This applies even when the
primary insurer would deny reimbursement
for the cost of the vaccine and its
administration because the plan’s
deductible has not been met.
Has health insurance covering all
vaccines, but has not yet met plan’s
deductible or paid for other services
received at visit and has Medicaid Title
XIX (19) as secondary insurance
Yes
Medicaid (V02)
Has health insurance covering all
vaccines, but the plan has a fixed dollar
limit or cap on amount that it will cover
Yes
Insured (V01) until the fixed dollar limit is
met.
Underinsured (V05
1
) after the fixed dollar
limit is reached.
Has an insurance plan that does not
cover all ACIP-recommended vaccines
Yes
Underinsured (V05
1
). Child can only receive
vaccines not covered by the plan.
Has health insurance, but plan does not
cover any vaccines
Yes
Underinsured (V05
1
). With implementation
of ACA, this situation should be rare.
Enrolled in CHIP – Title XXI (21) or State-
Funded
Not eligible
for VFC, but is
eligible for
CHIP vaccines
Insured (V22 CHIP). The VFC program
distributes vaccines for CHIP-covered
children. See section 4 for more information
on CHIP.
Has no health insurance coverage
Yes
Uninsured (V03)
Has private health insurance that covers
all vaccinations and is AI/AN
Yes
AI/AN (V04). However, the provider should
choose the eligibility category most cost-
effective for the child and family.
Has Medicaid Title XIX (19) and is AI/AN
Yes
Medicaid (V02) or AI/AN (V04). Providers
should use Medicaid for the administration
fee because this provides the least out-of-
pocket expense for the family.
1
VFC vaccines for the underinsured may only be administered by a federally qualified health center (FQHC), rural
health clinic (RHC), or a deputized local health department.

02/07/2020 Page 14 of 73
Child’s Insurance Status
VFC-Eligible?
VFC Eligibility Category
Enrolled in a Health Care Sharing
Ministry
Uninsured-
Yes
Insured-No
Underinsured-
Yes
1
Depends if the plan is recognized as an
insurance plan and if the insurance plan
covers vaccines:
• If the plan is NOT recognized by the
state insurance department as
insurance, then the child is uninsured
(V03), regardless of vaccine coverage
provided by the plan, and eligible for
VFC.
• If the plan is recognized by the state
insurance department and the plan
covers vaccines, the child is insured
(V01) and not eligible for VFC vaccines.
• If the plan is recognized by the state
insurance department but the plan does
not cover all ACIP-recommended
vaccines, the child is underinsured (V05)
for the vaccines not covered by the
insurance
1
.
The chart below summarizes the type of vaccines to be used on patients with Medicaid Title XIX (19)
coverage.
THE PATIENT’S AGE
IS:
VFC VACCINES
Eligible for VFC vaccines.
Bill HFS for Admin Fee.
PRIVATELY PURCHASED VACCINES
Administer privately purchased vaccines.
Bill HFS or plan for vaccine(s).
18 years or younger
Yes
No
19 years or older
No
Yes
INSURED CHILDREN WITH MEDICAID TITLE XIX (19) AS SECONDARY INSURANCE
Some children may have a private primary health insurance plan with Medicaid Title XIX (19) as their
secondary insurance. These children are considered VFC-eligible because of their Medicaid Title XIX (19)
enrollment. However, their parents are not required to participate in the VFC program.
Billing options exist for the parent and provider in this situation. The provider should choose the option
that is most cost-effective for the family. The parent of a child with Medicaid Title XIX (19) as secondary
insurance should never be billed for a vaccine or an administration fee.
Options include:
• Option 1: The provider can administer VFC vaccines and bill Medicaid for the administration fee.
Considerations regarding this option:
o Easiest way for a provider to use VFC vaccines and bill Medicaid for the administration fee
o No out-of-pocket costs to the parent for the vaccine or the administration fee
• Option 2: The provider can administer private stock vaccines and bill the primary insurance
carrier for both the cost of the vaccine and the administration fee. Considerations regarding this
option:

02/07/2020 Page 15 of 73
o The provider may be reimbursed a higher dollar amount if privately purchased vaccine is
administered and both the vaccine and the administration fee are billed to the primary
insurer.
MEDICAID AS SECONDARY INSURANCE AND HIGH-DEDUCTIBLE INSURANCE PLANS
If a child has Medicaid Title XIX (19) as secondary insurance and the primary insurance is a high-
deductible insurance plan requiring the parent to pay out of pocket for vaccines, the child should be
considered VFC-eligible (V02) if the family has not yet reached its deductible.
VFC vaccines should be administered, and the administration fee should be billed to Medicaid until the
deductible is reached.
If a child does not have Medicaid Title XIX (19) as secondary insurance, the child is considered insured
(V01) and not VFC-eligible even if a child’s family has a high-deductible plan.
UNDERINSURED
Underinsured means the child has health insurance, but the insurance policy:
• Doesn’t cover any ACIP-recommended vaccines;
• Doesn’t cover all ACIP-recommended vaccines (underinsured for vaccines not covered); or
• Does cover ACIP-recommended vaccines but has a fixed dollar limit or cap for vaccines.
The child is considered underinsured once the fixed dollar amount is reached.
Before administering a vaccine, providers must verify whether the child’s health insurance plan covers
ACIP-recommended vaccines. If the provider cannot verify vaccination coverage, for the purposes of the
VFC program, the child is considered insured (V01) and not eligible to receive VFC vaccines at that
immunization encounter. VFC vaccines for the underinsured may only be administered by a federally
qualified health center (FQHC), rural health clinic (RHC), or a deputized local health department.
HEALTH CARE SHARING MINISTRIES
Health Care Sharing Ministries (HCSMs) are nonprofit alternatives to purchasing health insurance from
private, for-profit insurers. Generally, HCSMs are organizations whose members share a common belief
system and collectively “share” the cost of their members’ medical care and are usually not considered
as an insurance plan. See the VFC Eligibility Scenario chart below for more information.
For the VFC program, “insurance” is defined as a plan that is:
• Regulated by a State’s Insurance Commissioner and/or
• Subject to the Employee Retirement Income Security Act of 1974 (ERISA), a federal law that sets
minimum standards for most voluntarily established pension and health plans in private industry
to provide protection for individuals in these plans.
The Illinois Department of Insurance regulates insurance plans in Illinois and may assist in determining if
a plan is insurance or a health cost-sharing plan. Contact information is available at
http://insurance.illinois.gov/main/contactUs.html
.

02/07/2020 Page 16 of 73
VFC ELIGIBILITY IN SPECIAL CIRCUMSTANCES
Special
Circumstance
Vaccination Service
Location
Child’s Insurance
Status
VFC-Eligible?
VFC
Eligibility
Seeking
contraceptive or
STD services
and wants to be
vaccinated
School-located clinic
or any VFC-enrolled
provider whose main
services are primary
or urgent care
For confidentiality
reasons, does not want
to use insurance
No
Insured
(V01)
Seeking
contraceptive or
STD services
and wants to be
vaccinated
Family planning clinic
or STD clinic
For confidentiality
reasons, does not want
to use insurance or
insurance status is
unknown
VFC-eligible;
however, eligibility
must comply with
the state’s medical
consent laws for
minors
Uninsured
(V03)
Incarcerated
Juvenile detention
center that does not
purchase vaccines
Lost access to health
insurance due to
incarceration
Yes
Uninsured
(V03)
STATE OF RESIDENCY
At times, VFC-eligible children receive health care in a bordering state instead of their state of residency.
VFC eligibility is not dependent upon state of residency for the child. Illinois providers enrolled in the
VFC program may vaccinate children through age 18 who are VFC-eligible residing in another state.
Providers must be aware if VFC vaccines are administered to a Medicaid Title XIX (19) VFC-eligible child
from a neighboring state, the provider must be a Medicaid-enrolled provider for the state where the
Medicaid Title XIX (19) VFC-eligible child resides to receive reimbursement for the administration fee
from that state’s Medicaid program.
PROVIDER RESPONSIBILITY TO SCREEN FOR VFC ELIGIBILITY
Screening to determine a child’s eligibility to receive vaccines through the VFC program must take place
with each immunization visit. The Patient Eligibility Screening Form developed by the Department
provides a means of recording parent response to VFC eligibility questions. The provider, parent, or
guardian may complete the VFC eligibility portion of the form. Verification of parent/guardian responses
is not required. Providers must correctly document VFC eligibility in I-CARE for each dose of vaccine
administered.
Providers using electronic medical records (EMRs) to document vaccinations must have the capability to
enter VFC eligibility status and include all criteria from the Patient Eligibility Screening Record.
Before administering vaccines at each immunization encounter, providers must check eligibility status
and type of Medicaid coverage in the MEDI system
(http://www.illinois.gov/hfs/MedicalProviders/EDI/medi/Pages/default.aspx
) or an equivalent system
receiving HFS 270/271 electronic transaction data.
VFC ELIGIBILITY DECISION TREE AND SCENARIO CHART
The following eligibility decision tree will assist in determining if a patient is eligible to receive VFC or
CHIP vaccines.

02/07/2020 Page 17 of 73
02/07/2020 Page 18 of 73
4. CHILDREN’S HEALTH INSURANCE PROGRAM (CHIP)
As of September 1, 2019, the Illinois VFC program started providing vaccines purchased by HFS for use
with children under the age of 19 with the CHIP coverage. CHIP coverage includes Title XXI [21] or State-
funded coverage and hereafter will be referred to as “CHIP.”
Children who have Medicaid “Title XXI [21]” or “State-funded” coverage (as shown in MEDI in the
“Special Information” section) are not eligible for VFC vaccines and must receive CHIP vaccines. These
children have CHIP coverage and are considered fully insured.
The chart below summarizes the type of vaccines to be used on patients with CHIP coverage.
THE PATIENT’S AGE IS:
CHIP VACCINES
Eligible for CHIP vaccines
through VFC.
Bill HFS for Admin Fee.
PRIVATELY PURCHASED VACCINES
Administer privately purchased
vaccines.
Bill HFS or plan for vaccine(s).
18 years or younger
Yes
No
19 years or older
No
Yes
More information on the CHIP program and MEDI is available on the HFS website at
https://www.illinois.gov/hfs/MedicalProviders/NonInstitutional/Pages/default.aspx
.
For questions regarding Medicaid or CHIP billing, please contact the Illinois Department of Healthcare
and Family Services, Bureau of Professional and Ancillary Services at 877-782-5565.
The CDC requires that all VFC programs determine individual provider populations served and
associated vaccine need by fund type. Illinois is required to establish a process for collecting and
validating provider populations to ensure publicly purchased vaccines are distributed in amounts
representing the provider population served and to adjustments if the population served changes.
VFC providers place orders to receive vaccines for CHIP-eligible children through the Illinois VFC
program. The number of vaccines a clinic will receive for their CHIP-eligible children will be dependent
on the patient population indicated in the VFC enrollment form in I-CARE.
VFC clinics are required to update their patient population at minimum annually or more often as
needed. VFC clinics may submit their CHIP population or updates to their CHIP population through the
online survey available at https://app.smartsheet.com/b/form/58f0005616e84e0cacf5a07821d22695
.
The VFC clinic’s patient population may be viewed in the current VFC enrollment form. In I-CARE, click
on the site tab and then go to VFC. Click on the enrollment button and select the current year’s
enrollment form. See the screen shot on the following page.

02/07/2020 Page 19 of 73
Scroll down until you get to the patient population section.
The percentage of CHIP vaccines the VFC clinic will receive is based upon the clinic’s patient population
and applies the same to all vaccines. For this sample clinic shown on the previous page, their largest
02/07/2020 Page 20 of 73
population is the group 1 to 6 years of age, with 1,492 VFC eligible children and 130 CHIP children
between 1 to 6 years of age.
For example, this clinic determined they need 10 doses of rotavirus vaccines for their next 1 to 3 months
of appointments. In their order of 10 doses, they will receive 9 doses of VFC and 1 doses of CHIP. The
clinic estimates 120 children will need measles, mumps, rubella vaccine in the next 1 to 3 months. In
their order of 120 doses, they will receive 108 doses of VFC and 12 doses of CHIP.
All vaccines will be split according to the clinic’s patient population, with the following exceptions.
• Single-dose packages of Bexsero, Pneumovax 23, and TD will default to VFC funding type. If a
VFC clinic needs one of these vaccines for CHIP, please enter a note in the “Status Comments”
box in the vaccine order form stating the name of the vaccine and specify the funding type
needed.
• ProQuad and Varivax vaccines are shipped frozen directly from the manufacturer, Merck. Merck
is unable to split funding sources on an order for ProQuad or Varivax and only one funding type
for frozen vaccines may be specified per order. Orders for ProQuad and Varivax will default to
VFC funding. If VFC clinics need CHIP-funded ProQuad or Varivax, the VFC clinic should enter a
note in the “Status Comments” box specifying either CHIP ProQuad or CHIP Varivax is needed.
If both VFC and CHIP doses are needed for ProQuad or Varivax, the VFC and CHIP orders must be
placed separately. The clinic should enter the separate order for either CHIP Varivax or ProQuad
and add a note to indicate the frozen vaccine order is needed for CHIP. The orders may be
placed only in multiples of 10.
All other vaccines are available only in the package sizes listed in I-CARE. Any order would be split
according to the VFC clinic’s patient population profile.
See section 9 for information on ordering and receiving vaccines and section 10 for information on
storing vaccines.

02/07/2020 Page 21 of 73
5. VACCINE STAFF AND TRAINING
VACCINE COORDINATORS
During the enrollment process, VFC providers are required to designate a primary vaccine coordinator
and at least one backup vaccine coordinator for each facility. The primary vaccine coordinator will be
responsible for ensuring all vaccines are stored and handled correctly and should be an expert in the
clinic’s storage and handling standard operating procedures (SOPs).
The vaccine coordinator is responsible for overseeing all vaccine management within the facility,
including:
• Developing and maintaining the Vaccine Management Plan
• Monitoring storage and handling and vaccine administration practices in the facility
• Ensuring and documenting annual vaccine management training for designated staff, as well as
training new staff upon hire
• Participating in and documenting completion of annual training on VFC requirements
• Storing all required documentation for three years, or longer if required by state statutes or
rules
The vaccine coordinator responsibilities include:
• Ordering vaccines
• Overseeing proper receipt and storage of vaccine deliveries
• Documenting vaccine inventory information
• Organizing vaccines within storage units
• Setting up temperature monitoring devices
• Checking and recording the current temperatures at the start and end of each workday
• Checking and recording minimum/maximum temperatures at the start of each workday
• Reviewing and analyzing temperature data at least weekly for any shifts in temperature trends
• Rotating stock at least weekly so vaccines with the earliest expiration dates are used first
• Removing expired vaccine from storage units
• Responding to temperature excursions (out-of-range temperatures)
• Maintaining all documentation, such as inventory and temperature logs
• Organizing vaccine-related training and ensuring staff completion of training
• Monitoring operation of vaccine storage equipment and systems
• Overseeing proper vaccine transport (when necessary) per SOPs
• Overseeing emergency preparations per SOPs:
o Tracking inclement weather conditions
o Ensuring appropriate handling of vaccines during a disaster or power outage
Coordinator responsibilities may be completed by the primary coordinator or backup coordinator
delegated. The primary vaccine coordinator must ensure the backup coordinator(s) are trained and
maintain documentation of competency for the specific task(s) assigned.
To effectively perform their duties, the vaccine coordinator and backup coordinator(s) must be fully
trained on routine and emergency standard operating procedures (SOPs) for vaccine ordering, storage,
handling, transport, and inventory management.
VFC providers are required to notify the Illinois VFC program anytime there is a change in vaccine
coordinator staff or the medical director.

02/07/2020 Page 22 of 73
STAFF TRAINING
All staff members who receive vaccine deliveries as well as those who handle or administer vaccines
should be trained in vaccine-related practices and be familiar with your clinic’s storage and handling
SOPs.

02/07/2020 Page 23 of 73
6. VACCINE STORAGE AND TEMPERATURE MONITORING EQUIPMENT
Vaccine management is a broad term intended to describe the storage and handling practices that
should be followed by all VFC providers. While the vaccine management practices here specifically
apply to vaccines provided through the VFC program, we recommend providers consider the VFC
vaccine management as a best practice for their private vaccine inventory as well.
The CDC Vaccine Storage and Handling Toolkit provides guidance and best practices for all health care
providers (including VFC-enrolled providers) and is available at
http://www.cdc.gov/vaccines/recs/storage/toolkit/storage-handling-toolkit.pdf
.
VACCINE COLD CHAIN
All VFC vaccine storage and handling requirements and recommendations are in place to ensure the
vaccine cold chain is maintained. The cold chain begins at the manufacturing plant, includes delivery to
and storage at the provider facility, and ends with administration of vaccine to the patient. Too much
exposure to heat, cold, or light at any step in the cold chain can result in a loss of vaccine potency. Once
potency is lost, it cannot be restored. Each time vaccines are exposed to improper conditions, potency is
reduced even further. With loss of potency, vaccines become useless and are unable to provide
immunity for the vaccinated individual.
Assuring vaccine quality and maintaining the cold chain are shared responsibilities among
manufacturers, distributors, public health staff, and health care providers.
An effective cold chain relies on three main elements:
• A well-trained staff
• Reliable storage and temperature monitoring equipment
• Accurate vaccine inventory management
Results of a cold chain failure can be costly. ACIP’s General Best Practice Guidelines for Immunization
states, “vaccine exposed to inappropriate temperatures that is inadvertently administered should
generally be repeated.”
2
A break in the cold chain can mean extra doses for patients, increased costs for providers, and damage
to public confidence in vaccines. More importantly, patients refusing revaccination can remain
unprotected from serious, vaccine-preventable diseases.
CDC’s Vaccine Storage and Handling Toolkit provides guidance on safe and effective vaccine
management practices for all health care providers. Though VFC providers are required by the VFC
program to implement the recommendations and best practice guidance in the CDC Vaccine Storage
and Handling Toolkit, the Illinois VFC program has additional requirements providers must adopt. The
requirements are described below. Following these requirements, recommendations, and best practice
guidance in the toolkit can minimize financial burden for providers due to vaccine loss and prevent the
need for revaccination. The result is maximum vaccine effectiveness and patient protection.
2
Centers for Disease Control and Prevention. ACIP’s General Best Practice Guidelines for Immunization,
https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html
.
02/07/2020 Page 24 of 73
Vaccine appearance is not a reliable indicator that vaccines have been stored in appropriate conditions.
For example, inactivated vaccines—even when exposed to freezing temperatures—may not appear
frozen, giving no indication of reduced or lost potency.
By following and implementing CDC-recommended storage and handling practices, providers can ensure
patients receive high-quality vaccine that has not been compromised.
REFRIGERATOR AND FREEZER UNITS
Storage units must have enough room to store the largest inventory a provider might have at the busiest
point in the year without crowding.
EQUIPMENT TYPES
CDC recommends the following units, in order of preference, for the storage of VFC vaccines:
• Purpose-built or pharmaceutical/medical-grade units, including doorless and dispensing units
• Stand-alone refrigerator and freezer units—these units can vary in size from a compact, under-
the- counter style to a large, stand-alone, pharmaceutical- grade storage unit
The Illinois VFC program does not allow combination household refrigerator/freezer units for the
storage of vaccines obtained through the VFC program.
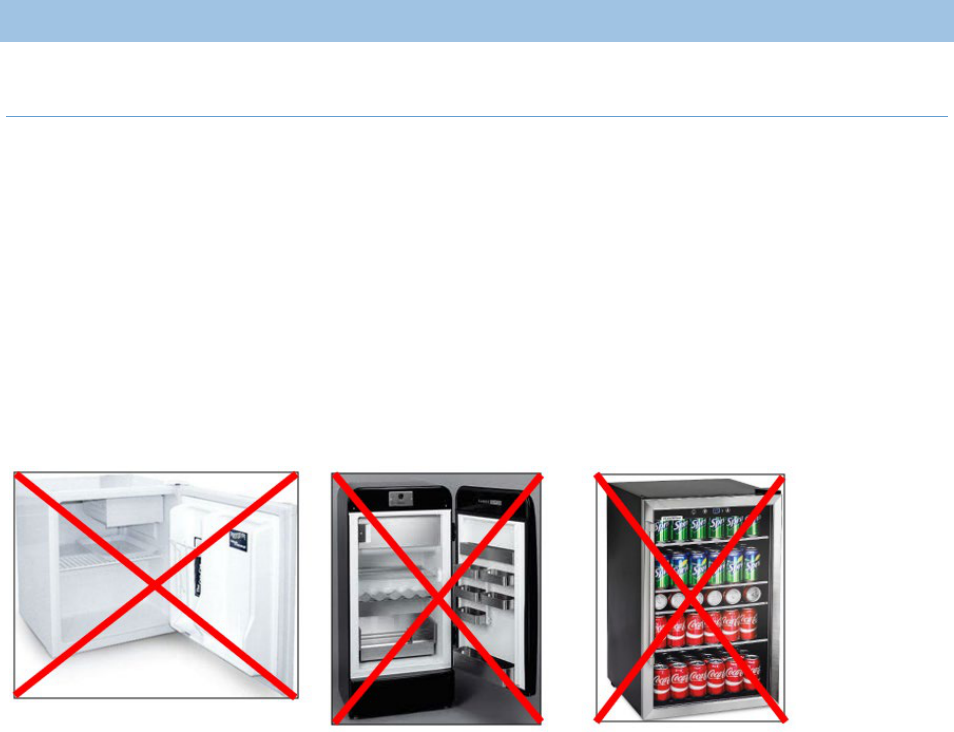
The use of dormitory or bar-style refrigerator/freezers is prohibited at all times for VFC program
providers. These units have a single exterior door and an evaporator plate/cooling coil, usually located
in an icemaker/freezer compartment. The following examples are dormitory-style or bar-style units and
are NOT allowable to store VFC vaccines at any time.
The following refrigerators are the size of a household refrigerator, but they are still classified as a dorm-
style refrigerator because they have the one exterior refrigerator door with the freezer compartment
located within the refrigerator sections. These are not allowable units for the storage of vaccines
obtained through the VFC program.

02/07/2020 Page 25 of 73
PURPOSE-BUILT VACCINE STORAGE UNITS
Numerous vaccine storage units have entered the market that are designed specifically for the storage
of vaccines. These purpose-built for vaccine storage can take many physical forms. Some look like
traditional standalone units, while others can take the form of dispensing or vending units either with or
without doors. Although these units may be similar to pharmaceutical grade or medical grade units, they
are unique in that they are designed and tested to keep vaccines at their appropriate storage conditions.
Purpose-built vaccine storage units must meet the same requirements as other VFC storage units.
• Temperature Monitoring
o Many purpose-built units have multiple temperature probes or sensors. It is important that
these probes or sensors have current Certificates of Calibration.
o Many of the purpose-built closed or doorless units may utilize air sensors (non-buffered
probes). Since these units have very limited exposure to ambient air, the use of a buffered
probe is not essential.
o Digital Data Logger – Many purpose-built units will have built-in data loggers with electronic
interfaces that will allow continuous temperature tracking and/or provide min/max
temperatures. Providers should ensure the purpose-built unit will meet the same
temperature monitoring device requirements as defined for other VFC storage units.
o VFC providers are required to monitor, assess and document temperatures on a paper log
with two current temperature readings per day, at least three days per week, at the
beginning of the day and prior to closing, and the minimum and maximum temperatures
documented at the beginning of each workday.
o All temperature documentation must contain the time and date of each reading and the
name (or initials) of the person who assessed and recorded the readings.
o Data logger temperatures must be downloaded and reviewed at least on a weekly basis.
Data logger files must be stored for at least three years.
• Vaccine Storage
o Many purpose-built units have undergone testing and temperature mapping to have the
probe placed in the most appropriate location.

02/07/2020 Page 26 of 73
o Although purpose-built units can have multiple temperature probes, a backup temperature
monitoring device is still needed for transport to a backup facility in an emergency.
o Many purpose-built units do not need water bottles to serve as thermal ballast.
• Vaccine Management
o Purpose-built units must have the ability to separate public and private vaccine stock either
physically or electronically.
o If stock is separated electronically, an inventory printout must be accessible upon request.
o If unable to physically remove expired vaccine from a purpose-built unit immediately, the
unit must be able to make expired vaccines inaccessible.
o The only NDC and lot number that can be used to order, report inventory, report
administered vaccines in I-CARE, or to submit vaccine returns is the NDC and lot number on
the outside box.
o In situations of a temperature excursion or power outage, the provider must ensure they
are able to remove and relocate the vaccines, if necessary, to an emergency response
location on their emergency response plan.
• Reporting Requirements
o VFC providers using the purpose-built dispensing units must ensure their unit is able to
produce reports listing inventory by funding type and data logger reports during annual
enrollment, during VFC site visits, or upon request.
o If vaccine stock is separated electronically, an inventory printout must list the public and
privately purchased stock by brand name, NDC, lot number and expiration date.
o If providers are unable to physically remove expired vaccine from a purpose-built unit
immediately after expiration, the unit must be able to make expired vaccine inaccessible.
An inventory printout must list the expired vaccines that are inaccessible.
STORAGE UNIT PLACEMENT
Good air circulation around the outside of the storage unit is important. Place a storage unit in a well-
ventilated room, leaving space between the unit, ceiling, and any wall. Nothing should block the cover
of the motor compartment. The unit should be firm and level, with the bottom of the unit above the
floor. Make sure the unit door opens and closes smoothly and fits squarely against the body of the unit.
If not secured properly, unit doors pose a particular risk to maintaining appropriate internal
temperatures of vaccine storage units. Studies find most units work best when placed in an area with
standard indoor room temperatures, usually between 20° C and 25° C (68° F and 77° F). Check the
manufacturer-supplied owner’s manual for additional guidance on placement and spacing.
STORAGE UNIT DOORS
A door that is not sealed properly or left open unnecessarily not only affects the temperature in a unit, it
also exposes vaccines to light, which can reduce potency of some vaccines. Consider using safeguards to
ensure the doors of the unit remain closed—for example, self-closing door hinges, door alarms, or door
locks.
STABILIZING TEMPERATURES IN NEW, MOVED, AND REPAIRED UNITS
It may take two to seven days to stabilize the temperature in a newly installed or repaired refrigerator
and two to three days for a freezer.
Before using a unit for vaccine storage, check and record the minimum and maximum temperatures and
the current temperatures two times a day on each workday for two to seven days. Once two

02/07/2020 Page 27 of 73
consecutive days of temperatures are recorded within the recommended range, the unit is stable and
ready for use.
TEMPERATURE RANGES
Refrigerators should maintain temperatures between 2° C and 8° C (36° F and 46° F). Freezers should
maintain temperatures between -50° C and -15° C (-58° F and +5° F). The Illinois VFC program
recommends setting temperatures in Celsius and recording temperatures to one decimal place (i.e. 4.2
C). Refrigerator or freezer thermostats should be set at the factory-set or midpoint temperature, which
will decrease the likelihood of temperature excursions.
Consult the owner’s manual for instructions on how to operate the thermostat. Thermostats are marked
in various ways and, in general, show levels of coldness rather than temperatures. The only way to know
the temperature where vaccines are stored is to measure and monitor it with a temperature monitoring
device.
DIGITAL DATA LOGGERS
VFC providers must use digital data loggers (DDLs) with continuous temperature monitoring capability
and a current and valid Certificate of Calibration Testing (also known as a Report of Calibration) in each
unit storing public vaccines. DDLs must be used during routine, on-site vaccine storage, vaccine
transport, and off-site clinics. The VFC program recommends having a backup data logger for each
emergency transport unit.
To meet VFC program requirements, the DDL must be equipped with:
• A temperature probe or sensor (a buffered probe is recommended);
• An active temperature display outside the unit that can be easily read without opening the
storage unit’s door; and
• Continuous temperature monitoring and recording capabilities and the capacity to routinely
download data.
Additional recommended DDL features include:
• Alarm for out-of-range temperatures
• Temperature display showing current, minimum, and maximum temperatures
• Low battery indicator
• Accuracy of +/-1°F (0.5°C)
• User-programmable logging interval (or reading rate) recommended at a maximum time interval
of no less frequently than every 30 minutes
Certificates of Calibration Testing must include:
• Model/device number
• Serial number
• Date of calibration (report or issue date)
• Confirmation the instrument passed testing (or instrument in tolerance)
The certificate of calibration testing must be issued by an appropriate entity. The certificate must
indicate at least one of the following items below about calibration testing.
• Conforms to ISO 17025
• Testing was performed by an ILAC/MRS Signatory body accredited laboratory.
• Is traceable to the standards maintained by NIST

02/07/2020 Page 28 of 73
• Meets specifications and testing requirements for the American Society for Testing and
Materials (ASTM) Standard E2877 tolerance Class F (0.5 °C) or better
If a VFC provider’s certificate(s) of calibration does not have all the required items, contact the
manufacturer of the data logger (or whoever did the calibration testing) to see if they will reissue the
certificates. Several manufacturers have indicated they are willing to reissue certificates to include the
missing items.
If a VFC provider needs to purchase new data logger, we recommend contacting the company and to
obtain a sample of their certificate of calibration to ensure all the required items are listed before
purchasing the data logger. If you would like for the Illinois VFC program to review a sample certificate
of calibration, please email it to [email protected]
. Please be sure to include your VFC PIN on
all communication.
A backup DDL must be readily available in case a DDL fails or calibration testing is required. The back-up
DDL should have a different calibration retesting date than other DDLs to avoid requiring all DDLs to be
sent out for recalibration at the same time. If the backup DDL has the same calibration retesting date,
providers must have the unit retested prior to expiration ensuring that a valid DDL is available for
required temperature monitoring. Each VFC provider must have a backup DDLs on site. Backup DDLs
should not be stored in the storage unit. This can result in conflicting temperature readings between the
backup and main DDLs, which can lead to potential confusion.
VFC providers must adhere to the following guidance:
• All data loggers must have a certificate of calibration that is current (up to two years since last
calibration testing or based on the manufacturer’s recommended re-testing timeline as
indicated on the certificate of calibration).
• Download and review data logger data files on a weekly basis.
Certain types of temperature monitoring devices have significant limitations and should not be used to
measure temperatures in a vaccine storage unit. These devices can be difficult to read and, because they
only show the temperature at the exact time they are checked, may fail to detect temperatures outside
the recommended range.
CDC and the Illinois VFC program do not recommend the following temperature monitoring devices:
• Alcohol or mercury thermometers, even if placed in a fluid-filled, biosafe, liquid vial
• Bimetal stem temperature monitoring devices
• Temperature monitoring devices used for food
• Chart recorders
• Infrared temperature monitoring devices
• Temperature monitoring devices that do not have a current and valid Certificate of Calibration
Testing
Some devices sold in hardware and appliance stores are designed to monitor temperatures for
household food storage. They are not calibrated and not accurate enough to ensure vaccines are stored
within the correct temperature range. Using these devices can pose a significant risk of damaging
vaccines.
POWER SUPPLY
Even with appropriate equipment and temperature monitoring practices in place, power disruption can
result in destruction of the entire vaccine supply. Precautions should always be taken to protect the
storage unit’s power supply.

02/07/2020 Page 29 of 73
• Plug in only one storage unit per electrical outlet to avoid creating a fire hazard or triggering a
safety switch that turns the power off.
• Use a safety-lock plug or an outlet cover to prevent the unit from being unplugged.
• Post “DO NOT UNPLUG” warning signs at outlets and on storage units to alert staff, custodians,
electricians, and other workers not to unplug units.
• Label fuses and circuit breakers to alert people not to turn off power to a storage unit.
• Use caution when using power outlets that can be tripped or switched off and avoid using:
o Built-in circuit switches (may have reset buttons)
o Outlets that can be activated by a wall switch
o Multioutlet power strips
VACCINE UNIT SETUP
The diagrams below shows how the vaccine storage unit should be setup.

02/07/2020 Page 30 of 73
7. MOBILE VACCINE CLINICS
Vaccine storage in mobile vaccine clinics must meet the same VFC storage unit requirements:
pharmaceutical/medical grade or stand-alone refrigerators and freezers permanently installed within
the mobile clinic. These units may be either under-the-counter or upright units depending on the need.
The mobile clinic should be plugged into the home site location to either generators or another power
source when the mobile clinic is not being used. The mobile clinic vaccine storage units are continuously
monitored by a data logger with temperatures manually checked two times a day and logged into I-
CARE. The mobile vaccine clinic is treated as another exam room within the VFC provider site that
happens to have wheels and a motor. The mobile vaccine clinic must be inspected as part of the VFC
compliance site visit. Illinois VFC provider’s mobile vaccine clinics may not transport Illinois VFC vaccines
to the city of Chicago or outside of the state of Illinois. Although the Illinois VFC program does not have
a residency requirement for VFC-eligible children, the VFC vaccines may only be administered by
providers within the Illinois VFC project area, which does not include the city of Chicago or other states.
The vaccines must be delivered to the VFC provider’s “brick and mortar” site, as with all the other VFC
vaccines. If vaccines are to be permanently stored in the mobile vaccine clinic, the mobile unit must
have a permanent source of power, either a generator or other permanent power source.
The following pictures shows an example of a mobile medical van.

02/07/2020 Page 31 of 73
8. OFF-SITE VACCINE CLINICS
VFC-enrolled providers may conduct temporary, off-site clinics. The transportation, storage and
handling of VFC-program vaccines must meet the guidelines in the program manual and in the CDC
Vaccine Storage and Handling Toolkit.
Current VFC policy specifies that VFC vaccines are to be delivered directly to VFC clinic location on file in
the current enrollment. The VFC vaccines may only be administered by providers within the Illinois VFC
project area, which does not include the city of Chicago or other states.
The VFC program and CDC does not recommend routine transport of vaccine due to the risk to the cold
chain and vaccine viability. However, because most temporary mass clinics typically require vaccine
transport on the day of the clinic, the VFC program and CDC has determined that these temporary off-
site clinics (e.g., school located clinic) require enhanced storage and handling practices.
The total time for transport alone or transport plus clinic workday should be a maximum of 8 hours (e.g.,
if transport to an off-site clinic is 1 hour each way, the clinic may run for up to 6 hours).
3
Only the
amount of vaccines that are needed for the workday should be transported to each scheduled clinic.
See section 9 for details on storing vaccines and section 10 for details on transporting vaccines and
transport system recommendations.
If frozen vaccines must be transported, use a portable vaccine freezer unit or qualified container and
packout that maintains temperatures between -50° C and -15° C (-58° F and +5° F)
4
. Immediately upon
arrival at the destination, unpack the vaccines and place them in a freezer at a temperature range
between -50° C and -15° C (-58° F and +5° F). Any stand-alone freezer that maintains these temperatures
is acceptable.
Temporary off-site clinic vaccine storage must meet VFC program requirements to maintain appropriate
temperatures throughout the clinic day and temperatures monitored with a digital data logger during
transport and during storage at the off-site clinic. See section 10 for more information on storing
vaccines.
At the end of the temporary off-site clinic, the vaccines must be transported back to the VFC provider’s
permanent location in the approved transport method. Providers must review the data logger data file
to verify the vaccines were stored and transported within proper temperature ranges before returning
the vaccines to the clinic’s permanent inventory to prevent administration of vaccines that may have
been compromised. Vaccines exposed to temperature excursions must be labeled “do not use” until
further information can be gathered from the manufacturer(s) and verified by IDPH on the usability of
the vaccine. See section 10 for more information on temperature excursions.
VFC-enrolled providers must submit an off-site vaccine clinic notification form in I-CARE at minimum 48
hours prior to the event. Only one event may be submitted per notification form.
3
CDC Vaccine Storage and Handling Toolkit pages 21-22.
http://www.cdc.gov/vaccines/recs/storage/toolkit/storage-handling-toolkit.pdf
.
4
CDC Vaccine Storage and Handling Toolkit pages 23. http://www.cdc.gov/vaccines/recs/storage/toolkit/storage-
handling-toolkit.pdf.
02/07/2020 Page 32 of 73
VFC-enrolled providers must provide the following information in the off-site vaccine clinic notification
form.
• The VFC provider submitting notification of the event.
• The VFC coordinator name and contact information who is submitting the notification.
• List any partners involved in the off-site clinic, including other VFC-enrolled providers and non-
VFC providers.
• All non-VFC providers must sign the VFC Provider Agreement to be uploaded with the
notification form.
• The VFC provider submitting the event notification must submit a written description detailing
the responsibilities for each non-VFC party involved.
• List the date, location, target population, and vaccines to be provided at the off-site clinic.
The VFC coordinator will submit the off-site clinic notification by checking a box indicating agreement
with the Vaccines for Children storage and handling requirements as listed in the Illinois Vaccines for
Children Program Manual and the CDC Storage and Handling Toolkit and understanding the medical
director is accountable for compliance with these requirements.
The VFC provider should maintain a copy of the off-site clinic notification form in their records.

02/07/2020 Page 33 of 73
9. ORDERING AND RECEIVING VACCINES
PLACING VACCINE ORDERS
Providers should order vaccine in accordance with actual vaccine need for one month and avoid
stockpiling or build-up of more than a three-month supply. Providers should maintain enough vaccine
inventory to last one month; however, inventory should never exceed three months. Orders may take
two to three weeks from submission of order to vaccine delivery. Vaccines provided through the VFC
program must be distributed directly to the location at which the provider will administer the vaccines.
5
CDC recommends smaller, more frequent orders rather than large orders to minimize the amount of
vaccine loss if an incident occurs during shipment or in the vaccine storage unit. Storing a larger volume
of vaccines that a VFC provider needs can increase the risk of wasting vaccines if they expire before they
can be used or compromised in some way (e.g., due to mechanical failure of a storage unit).
All vaccine orders are submitted through I-CARE. Providers must ensure the following information is
completed or updated prior to submitting an order in I-CARE:
• Patient immunization records showing how each dose of VFC vaccine was administered.
• Temperature logs for all appliances are up-to-date as of the date the order is requested.
• All data logger certificates of calibration are valid and not expired.
• All temperature excursions must have a vaccine incident report on file.
• No expired vaccines are showing in the clinic’s inventory.
• The clinic’s inventory in I-CARE matches the physical inventory.
• The clinic’s inventory in I-CARE is not showing any negative balances.
• Clinic must be open at least three days a week with at least four consecutive hours a day to be
able to receive a delivery. Delivery hours must be entered and updated in I-CARE, including
specifying if the clinic is closed during lunch or other hours, when placing orders through I-CARE.
• The vaccine order is enough for at least one month’s inventory but does not exceed three
months.
VFC providers should consider their clinic’s delivery hours for the next two to three weeks to ensure a
VFC vaccine coordinator will be on site to accept the delivery before placing an order.
The “Status Comment” field in the I-CARE order form should not be used to convey any of the following:
• Open/closed days
• Open/closed hours
• Critical delivery information
All Illinois VFC providers must provide individual patient immunization records on how each VFC vaccine
was administered. The individual patient immunization records can either be directly entered into I-
CARE or can be electronically transmitted to I-CARE from the provider's electronic medical record (EMR)
system. VFC providers not in compliance will not be able to continue participating in the VFC program.
Providers interested in setting up their EMR to transmit data to I-CARE should contact the I-CARE team
.
5
Centers for Disease Control and Prevention. NCIRD Policy Regarding Grantee-supported Vaccine Depots

02/07/2020 Page 34 of 73
Providers must notify the VFC program when there has been a change in the VFC coordinator, medical
or use the “Contact
Us” button in I-CARE and select “VFC Illinois” under the category for additional assistance.
PATIENT POPULATION PROFILES
The provider patient population profiles will be used by the Illinois VFC program to monitor provider
orders. The patient population profile is automatically populated in I-CARE based on the patient
immunization records entered by the clinic in I-CARE or has transmitted from the provider’s EMR.
Providers ordering more vaccine than should be needed for their VFC population will be contacted. If
orders for excessive amounts of vaccine are placed on a regular basis, the provider will be contacted.
The provider may be required to replace wasted vaccines due to excessive ordering.
TRACKING VACCINE ORDERS
Providers may track the status of the order in I-CARE.
• The vaccine line status will state the current status of the vaccine order.
o Requested: The order has been submitted to the Illinois VFC program.
o Approved: The Illinois VFC program has approved the vaccine requested.
o Transmitted: The order has been transmitted to CDC for processing.
o Shipped: The vaccine order has been shipped by either McKesson or Merck (frozen
vaccines).
o Completed: The vaccine order is complete.
• Once an order has shipped, VFC providers may go into the order and click on the symbol next to
the NDC number to expand the details row. The shipment tracking number will be listed.
Providers may click on this tracking number to go to the shipper’s website and get more
information, including signing up for delivery alert messages.
BORROWING VACCINES
VFC-enrolled clinics are expected to maintain adequate inventories of vaccine for their privately insured,
CHIP and VFC-eligible patients. Vaccines provided through the Illinois VFC program cannot be used to
replace a clinic’s privately-purchased vaccine inventory. The clinic must ensure their vaccine supply is
adequate to meet the needs of the VFC-eligible or CHIP-eligible patients.

02/07/2020 Page 35 of 73
VFC clinics may not swap doses between VFC inventory and CHIP inventory.
The VFC program does not allow the borrowing of VFC or CHIP vaccines. Private vaccines used on VFC
patients cannot be paid back using VFC vaccine. VFC and CHIP vaccines cannot be used in non-eligible
children and then paid back with private vaccine stock.
If a VFC clinic finds they need couple of doses of CHIP vaccines in between vaccine orders, VFC clinics
may check with other nearby VFC clinics to see if they could transfer the needed CHIP doses. Be sure to
fill out and submit the transfer request form, which is available in I-CARE.
If a VFC clinic runs out of vaccines during a clinic (whether it is VFC or CHIP) and is unable to find a clinic
to transfer the needed doses immediately, the clinic would need to reschedule any children until a
vaccine order can be placed and received. The VFC program does not allow the borrowing or swapping
of vaccines between VFC and CHIP.
RECEIVING AND UNPACKING VACCINE SHIPMENTS
Proper vaccine inventory management is essential for appropriate vaccine ordering and stock rotation,
and ensures your facility has the vaccines your patients need. Vaccines are expensive, so making sure
they are unpacked, stored, prepared, administered, and transported correctly is critical.
Maintaining the cold chain is the first step in vaccine inventory management. Staff members who might
accept vaccine deliveries should be trained to immediately notify the vaccine coordinator or alternate
coordinator when deliveries arrive. Vaccines must always be immediately checked and stored properly
upon arrival.
Vaccines and diluents must be carefully unpacked, stored at recommended temperatures, and
documented immediately after they arrive. Do not place an unopened and/or unpacked shipment box
in a vaccine storage unit because the cool packs shipped with the vaccine may make the packaged
vaccine too cold if placed inside the storage unit.
Immediately examine shipments for signs of damage and to guarantee receipt of the appropriate
vaccine types and quantities.
• Examine the shipping container and vaccines for signs of physical damage.
• Check the contents against the packing list to be sure they match.
• Check the order received against the order placed in I-CARE to ensure all vaccines ordered were
received.
• The frozen vaccine packing list will show the maximum time vaccines can be in transit based on
shipment date.
• If the shipment includes lyophilized (freeze-dried) vaccines, make sure they came with the
correct type and quantity of diluents.
• Immediately check both vaccine and diluent expiration dates to ensure you have not received
any expired or soon-to-expire products.
• Immediately check the cold chain monitor (CCM), a device used to monitor vaccine
temperatures during transport, if one was included, for any indication of a temperature
excursion during transit.
WITHIN TWO HOURS OF VACCINE DELIVERY: If any problem is noted with the delivery such as damage,
excessive shipping time, cold chain breach has occurred, or a delivery shortage is noted, VFC providers
must IMMEDIATELY call the Illinois VFC Program Services Staff at 217-785-1455.

02/07/2020 Page 36 of 73
• If the provider does not call the Illinois VFC program within two (2) hours of the vaccine delivery
to report discrepancies and/or cold chain issues, this constitutes provider negligence in
accordance with the Vaccine Loss and Replacement Protocol due to handling and storage
mishaps by provider staff. Shipments that result in vaccine loss negatively impact the Illinois
VFC vaccine budget.
• Providers should never refuse a shipment. Providers should receive the package and
IMMEDIATELY report any concerns to the Illinois VFC program. Shipments refused at the
provider site are not able to be returned and evaluated in a timeframe that is possible to save
the vaccines. Providers will be responsible for replacing any vaccines wasted due to refusal to
accept a shipment.
• When calling the Illinois VFC program about a vaccine delivery, staff will have to report on
temperature indicators if anything is wrong (cold chain breach indicated). CDC or McKesson
may ask for pictures of the vaccines received, including the shipping box. A questionnaire will
be completed with the Illinois VFC program, CDC, and/or the vaccine manufacturer to determine
viability. Provider staff should store the vaccine appropriately, mark the vaccines as “DO NOT
USE” until advised by the Illinois VFC program, and maintain the shipment packing list. Ensure
that temperature logs are maintained for the vaccine in question. IDPH, CDC, and/or McKesson
Specialty MAY ask for this paper work.
MERCK FROZEN SHIPMENTS
Shown below are examples of Merck frozen shipping containers.
Frozen vaccines are shipped directly from Merck and will contain a shipper insert in the box to let the
provider know how long the product is good for based on the shipment date shown on the packing list.
Shown below are examples of the one, two, and four-day shipper inserts.
With frozen vaccine
shipments, the diluent is located in the lid compartment of the shipping box.
IDENTIFYING THE VACCINES BY FUNDING TYPE
When the vaccine shipment is received, VFC providers will need to identify the VFC, State, CHIP, and 317
doses within the shipment.

02/07/2020 Page 37 of 73
The VFC program has four different funding sources for vaccines.
• VFC: Vaccines for use with VFC-eligible children only.
• VFC/State: State purchased vaccines for use with VFC-eligible children only.
• CHIP: Vaccines for use with CHIP covered children only.
• 317: Vaccines available for local health departments for use with 317-eligible adults or for
approved outbreak response.
VFC clinics have two ways to determine the number of doses by funding type.
The first way is to check the order in I-CARE. Go to the order in I-CARE. In the “Detail” column, click on
the symbol next to the NDC number to expand the details row.
When the details row is expanded, the split by funding type will be listed. This provider’s split is 7 doses
VFC/State and 3 doses CHIP. The State-purchased vaccines are part of the VFC inventory and to be used
with VFC eligible children.
In this order, the provider received 28 doses of VFC/State and 12 doses of CHIP of Pediarix.
The second way is to check the packing slip when the vaccine order is received. The packing slip shows
how the vaccine order was split between funding sources.

02/07/2020 Page 38 of 73
For this VFC clinic’s vaccine shipment, the packing slip shows 28 doses are State and 12 doses are CHIP.
The state doses are for VFC-eligible children and these vaccines would be combined with the VFC
inventory. In I-CARE, these are listed as VFC/State.
IDENTIFYING THE SPLIT DOSES
Vaccines must be stored in their original packaging with lids closed until ready for administration. Vials
and manufacturer-filled syringes should always be stored in their original packaging. Loose vials or
syringes may be exposed to unnecessary light, potentially reducing potency, and may be more difficult
to track for expiration dates. Removing doses from their original box may also impact inventory
management and increase the risk of administration errors because they may be confused with other
vaccines.
When more than one fund type is in a box, the VFC clinic must label the box to indicate the number of
doses by funding type. Sample labels are available for printing and may be found on the home page of I-
CARE under News and Announcements.
Using the previous order as an example, the provider received 40 doses or 4 boxes of vaccines: 28 doses
are VFC/State and 12 doses are CHIP.
Of the 28 doses VFC/State, 20 doses are from 2 full boxes. These should be marked as VFC. 8 VFC/State
doses will need to come out of another box.
Of the 12 doses CHIP, 10 of these are from 1 full box. This box should be marked as VFC. 2 CHIP doses
will need to come out of another box.
In the final box, you will have 8 doses of VFC/State and 2 doses CHIP. Using a label, highlight the
number of doses by funding type and apply the label to the box. As doses are administered, cross off
the doses on the label.
VFC
VFC
02/07/2020 Page 39 of 73
Here are additional tips on storing split fund boxes.
• When storing split fund boxes, it is helpful to:
o Store these vaccines in a separate bin
o Identify the bin as “split” so staff will know the vaccine is split between different funding
sources
• To keep track of your use:
o Label the box of single-dose or multi-dose vaccines
o Highlight the # of doses from each funding type
o Mark off vaccines administered by funding type as it is used
• Some other important tips to note:
o Do not cover important information such as vaccine name and lot# with your labels
o Always keep vaccines in their original packaging
o Ensure the short-dated vaccines are being used first before beginning another box

02/07/2020 Page 40 of 73
10. INVENTORY MANAGEMENT
STORING VACCINES
VFC clinics must develop a method for maintaining storing the vaccines to ensure VFC and VFC/State
doses are only used for VFC eligible children and CHIP doses are only used for CHIP-eligible children.
Clinics may decide to use separate storage units or maintain inventory in one unit. A separate
refrigerator is not a requirement.
If a VFC clinic uses separate units for CHIP vaccines, only full boxes of vaccines may be stored separately.
Any boxes with doses split for VFC, VFC/State, and CHIP must remain in the original box.
Here are two visual examples on how to store vaccines when you have full boxes with only one fund
type and boxes with split fund types.
VACCINE STORAGE WITH ONLY ONE FUND TYPE IN A BOX
Organize your storage unit so vaccines are separated by VFC and VFC/State, CHIP (also referred to by
CDC as “Other Public”), and private vaccines.
VACCINE STORAGE WITH MORE THAN ONE FUND TYPE IN A BOX
Vaccines must be kept in the original box.
When storing split fund boxes:
• store these vaccines in a separate bin
• Identify the bin as “split” so staff will know the vaccine is split between different funding sources
• Always keep vaccines in their original packaging
• Ensure the short-dated vaccines are being used first before beginning another box

02/07/2020 Page 41 of 73
VACCINE MANAGEMENT
DAILY TASKS
• When the clinic opens and before the clinic closes, read and record the current temperature for
each refrigerator and freezer storing VFC vaccines—even when using a continuous temperature
monitoring device/data logger.
• When the clinic opens, read and record the minimum and maximum temperature for each
refrigerator and freezer storing VFC vaccines, even when using a continuous temperature
monitoring device/data logger.
• Document temperatures on VFC temperature logs.
• The temperature logs must contain the time and date of each reading and the name or initials of
the person who assessed and recorded the reading.
• If out of range temperatures are noted, immediately quarantine the vaccines, download the
temperature data files from your data logger, and follow the guidance on the VFC Vaccine
Incident Report (available in I-CARE).
WEEKLY TASKS
• Ensure temperatures were recorded twice daily, staff printed names and initials, and corrective
actions were taken on any out of range temperatures.
• Download and analyze the data logger data files weekly to look for temperature trends that
might indicate performance issues or any out of range temperatures with vaccine storage units
and follow up on any out of range temperatures.
Note: All VFC program related documentation, including eligibility screening, data logger data files,
and vaccine order documentation, must be retained for three years.
• Enter the current, minimum, and maximum temperatures into I-CARE.
MONTHLY TASKS (OR MORE OFTEN AS NEEDED)
• Conduct a careful and accurate physical vaccine inventory and compare the physical inventory
to the inventory in I-CARE.
• Check vaccine expiration dates and rotate stock to place vaccines that will expire soonest in
front of those with later expiration dates.
• Transfer vaccines that will expire within six months to other providers (refer to the VFC Vaccine
Transfer Approval Request Form in I-CARE for information and guidance on obtaining IDPH pre-
approval).
• Remove any expired vaccines from the storage unit and enter the expired vaccine transaction
into I-CARE to receive a mailing label to return the vaccines to McKesson.
ANNUAL TASKS (OR MORE OFTEN AS NEEDED)
• Check the expiration dates on the certificates of calibration for all data loggers and backup data
loggers. See the data logger tracking form in the appendix to record the dates of calibration.
• Before the expiration date, arrange to have the data loggers recalibrated or purchase new data
loggers. The VFC program will allow two years from the calibration date or longer based on the
manufacturer’s recommended re-testing timeline as indicated on the certificate of calibration.
Note: If choosing to have your loggers recalibrated, backup data loggers will need to be placed in
each unit storing VFC vaccines while the primary data loggers are being recalibrated. The VFC
program recommends have the primary and backup data loggers calibrated on different schedules.

02/07/2020 Page 42 of 73
• File certificates of calibration in a readily accessible area, keep them for three years, and present
them to VFC program staff for review upon request.
• Review with key practice staff the vaccine management plan’s section on preparing for and
responding to vaccine-related emergencies.
ROUTINE MAINTENANCE
• Establish a regular routine for cleaning vaccine storage units. Regular maintenance is
recommended to ensure proper operation, to maintain required temperatures, and to extend
the useful life of the appliance.
• Maintenance of the refrigeration unit and freezer includes:
o Check the storage unit door seals regularly for signs of wear and tear. Seals should not be
torn or brittle and there should be no gaps between the seals and the body of the unit when
the door is closed. If seals need to be replaced, contact a repair technician immediately.
o Check door hinges and adjust so that the door opens and closes smoothly and fits squarely
against the body of the unit.
o Clean unit coils and motor. Dust and dirt buildup can affect transfer of heat from the coils
and prevent the unit from working efficiently.
o Clean inside of units to discourage bacterial and fungal growth. Cleaning must be done
quickly to minimize the risk of the temperature going out of range.
o Defrost manual-defrost freezers when the frost exceeds either 1 cm or the manufacturer’s
suggested limit. Follow the manufacturer’s instructions. While defrosting, store vaccines
temporarily in another unit with appropriate freezer temperatures.
• Keep a logbook (see the vaccine management plan) to indicate the date(s) of routine
maintenance tasks, date(s) of any repairs or servicing, and the name of the person and/or
company performing each of these tasks.
• Replace batteries in data loggers every six months, if batteries are accessible.
• If applicable, test backup generators quarterly and service backup generators at least annually
(check manufacturer specifications for test procedures and maintenance schedules).
• If your facility has a backup battery power source, it should be tested quarterly and serviced
annually (check the manufacturer’s guidance for testing procedures and maintenance
schedules).
BEST PRACTICES
The following are recommended practices for providers handling vaccines:
• Store vaccines in their original packaging
• Store vaccines in the middle of the unit, with space between both the vaccines and the
side/back of the unit.
• Do not store vaccines in the doors, vegetable bins, or floor of the unit, or under or near cooling
vents.
• Do not store food or drink in vaccine storage units.
• Place water bottles throughout the refrigerator and frozen water bottles in the freezer storage
units to:
o Stabilize or extend temperatures during a power outage,
o Help to mitigate the effects of frequent open/closing door during busy clinic days, and
o Serve as physical blocks preventing the placement of vaccines in areas of the unit that
are at higher risk for temperature excursions.

02/07/2020 Page 43 of 73
• Rotate vaccines every week or when a new shipment comes in so newer vaccines are stored
toward the back of the unit, while those soonest-to-expire are stored in the front and
administered first.
• Open only one vial or box of a vaccine at a time to control vaccine use and allow easier
inventory control. On each opened vaccine vial, indicate on the label the date and time it was
reconstituted or first opened.
• Store vaccine products that have similar packaging in different locations in the storage unit to
avoid confusion and medication errors.
• Limit access to the vaccine supply to authorized personnel only.
• Install locks on refrigerators and, if possible, the electrical plug.
• Safeguard public vaccines by providing facility security, such as temperature alarms and
restricted access to vaccine storage and handling areas.
• In regular clinics/practices, vaccines should be prepared immediately prior to administration.
CDC strongly recommends NOT pre-drawing doses before they are needed.
TEMPERATURE EXCURSIONS
Temperature excursions or inappropriate storage conditions for any vaccine require immediate action.
Any temperature reading outside the recommended ranges in the manufacturers’ package inserts is
considered a temperature excursion. In general, manufacturers analyze information about the
magnitude of the temperature excursion and the total amount of time that temperatures were out of
range, as well as information about the vaccine in question, to determine whether a vaccine is likely to
still be viable.
Any staff member who hears an alarm, notices a temperature excursion, or vaccine storage and
handling issue potentially affecting the viability of the vaccines must notify the primary or backup
vaccine coordinator immediately. Take immediate action as soon as temperature excursions are
identified with vaccines provided through the VFC program. Vaccine that is considered spoiled because
a provider did not take immediate or appropriate action on out-of-range temperatures may require the
provider to replace the wasted VFC vaccine dose-for-dose with private purchase vaccines according to
the VFC Vaccine Loss and Replacement Policy.
The Vaccine Incident Report is available in I-CARE and serves as a record of the incident, the steps taken
to determine vaccine viability, and the disposition of the affected vaccine. Keep a copy of this report in
your records.
If there is any question about whether vaccines may have been exposed to out-of-range temperatures
for any reason, CDC recommends the following steps:
1. Do not use or discard the affected vaccines until the vaccine viability has been determined by
the manufacturers and you have contacted the Illinois VFC program.
2. Label exposed vaccines, “DO NOT USE,” marking the boxes with an “X,” and isolate them from
other vaccines in the storage unit at the proper storage temperature.
3. The primary or backup vaccine coordinator, supervisor, or, if necessary, the person reporting the
problem should document the event on the Vaccine Incident Report and submit it to the Illinois
VFC program.
FREEZER DEFROST CYCLES AND TEMPERATURE EXCURSIONS
Freezers with automatic defrost may produce temperature excursions when going through defrost
cycles. Any time a vaccine storage unit has temperature excursions, a vaccine incident report must be
completed to follow up on the out of range temperatures, including temperature excursions from

02/07/2020 Page 44 of 73
defrost cycles. Merck has stated providers should contact them each time they have a temperature
excursion with frozen vaccines – even when it is due to defrost cycles. Merck explained the stability
information they provide is based upon the specific set of conditions the provider reports and should
not be applied generally across the board.
The CDC Storage and Handling Toolkit provides storage best practices that may help prevent
temperature excursions in freezers with the automatic defrost cycles:
• The vaccines and the data logger probe should be placed in the center of unit, 2 to 3 inches
away from walls, ceiling, floor, and door to allow the cold air to circulate. A data logger probe
placed near the walls, floor, vent, ceiling, or door may indicate temperatures that are warmer
during defrost cycles than the actual vaccine temperature.
• Frozen water bottles in the unit will help stabilize or extend temperatures in the freezer. Place
frozen water bottles against the walls, in the back, on the floor, and in the door racks. Putting
frozen water bottles in the unit can help maintain stable temperatures caused by frequently
opening and closing unit doors, power failures, or even the automatic defrost cycles. It can also
prevent vaccines from being stored in areas where there is a greater risk of out-of-range
temperatures (such as the floor and door).
For manual defrost freezers: While manually defrosting the freezer, providers should move their frozen
vaccines to another freezer that is being monitor with a DDL and temperatures documented. This
second freezer cannot be a household/commercial “combination” unit; it must be a stand-alone freezer
or pharmaceutical grade freezer. When the original freezer is once again maintaining stable
temperatures, the vaccines can be returned to the original unit.
PROVIDER-TO-PROVIDER TRANSFER OF VACCINES
CDC and the VFC program discourage regular transport of vaccines. The VFC program prefers that
vaccines remain at the original location where they were initially delivered to avoid a possible break in
the cold chain rendering the vaccine non-viable.
Where practical, and as long as the cold chain is maintained, transfer of short-dated vaccine can occur
between VFC providers to avoid wasting vaccine.
• The Illinois VFC program must review and approve all requests to transfer vaccines BEFORE the
transfer occurs.
• The Illinois VFC program requires the use of a data logger with continuous monitoring and
recording capabilities during transport of vaccines.
• All data loggers should have a current and valid certificate of calibration.
• The VFC program does not recommend or find acceptable the use of alternative, one-time use
temperature indicators since they do not provide adequate data on excursions that may occur
during transport.
TRANSPORT OR SHIPPING
The terms “transport” and “shipping” have different meanings although often used interchangeably.
• Transport involves the movement of vaccine over a short time and distance between providers.
• Transport is typically performed by providers using private vehicles or courier services.
• The expected length of transport is less than eight (8) hours or regular business day.
• The VFC program’s expectation is that transporting vaccines should be an extremely rare
occurrence.

02/07/2020 Page 45 of 73
• Shipping, as compared to transport, typically involves further distance and time to move vaccine
between locations.
• Often, vaccine is moved using a large, shipping management service and requires adherence to
shipping standards that go beyond CDC guidance for the transport of vaccine.
• The VFC program does not allow providers to ship vaccines due to the potential risks to the cold
chain and ultimately the viability of the vaccine.
TRANSFER PROCEDURE
Providers who have excess vaccine on hand that will not be used in three to six months before
expiration are encouraged to transfer this vaccine to other Illinois VFC providers to utilize, and thus
avoid being charged for wasted vaccine. Providers should begin this process approximately six months
of the vaccine expiring and until the vaccine expired. It is the provider’s responsibility to find another
provider willing to accept the vaccine, and to properly pack and transport the vaccines following
standard cold-chain procedures. VFC providers are not required to accept a transfer from another VFC
provider. Providers must allow up to 10 business days for transfer approval requests to be reviewed.
See the VFC Vaccine Transfer Approval Request Form in I-CARE for more information.
Transfers should only occur for the following reasons:
• Vaccine is six months or less from outdate, and unable to be used by provider.
• Area outbreak resulting in unexpected surge of walk-in patients.
• Clinic closure requiring redistributing vaccines to other VFC providers.
• Seasonal clinic needing to transfer vaccine to other VFC providers at the end of time facility will
be open.
The following transfer requests will be reviewed on a case-by-case basis with appropriate explanation
provided for the transfer request:
• Vaccines are more than six months from the expiration date.
• The provider has an immediate need for a couple of doses of vaccine before an order could be
received.
Providers may not transfer influenza vaccine. If a provider needs a vaccine, they may order the vaccine
as vaccine orders are usually shipped sooner than the 10 business days it could take to approve a
transfer of vaccines. Transfers should be done on a rare basis and only for the reasons stated above.
Vaccines should remain with the original location it was delivered to if possible, to avoid a possible
break in the cold chain rendering the vaccine non-viable.
Providers must obtain pre-approval from IDPH before any transfers. All transfer requests must be
submitted by and received by one of the VFC vaccine coordinators on file in the provider’s enrollment.
Transporting vaccines due to an emergency response and in accordance with your emergency response
plan is not a transfer and does not require pre-approval. These vaccines are temporarily being stored at
the emergency response location until the vaccines can be moved back to the original provider. If the
vaccines will not return to the original provider after the emergency response, the provider must submit
a transfer request.
The VFC provider requesting to transfer vaccines MUST advise the receiving VFC provider of all
temperature excursions affecting the vaccines and provide the receiving VFC provider with a copy of the
vaccine incident report with the manufacturer stability statements.
If a provider cannot be located to accept transferred vaccine, document attempted contacts on the
vaccine transfer contact log available in I-CARE on the home page under “Immunization Links.” If the

02/07/2020 Page 46 of 73
vaccines must be wasted, email or fax the completed vaccine transfer contact log to IDPH for review and
consideration in the vaccine replacement decision. It is not required to document all contacts about
transferring vaccines. However, if vaccines must be reported as expired, we will consider attempts to
transfer the vaccines in the vaccine replacement decision.
VACCINE TRANSPORTATION GUIDELINES
Vaccine Transportation Recommendations
• CDC discourages regular transport of vaccines. The VFC program prefers that vaccines remain at
the original location where they were initially delivered to avoid a possible break in the cold
chain rendering the vaccine non-viable.
• The shipment of vaccines by a provider through a commercial carrier is not allowed due to the
potential risks to the cold chain.
• Providers must maintain the vaccine cold chain at all times to protect the vaccine potency.
• If you cannot ensure the vaccine has been stored under proper conditions to maintain the cold
chain, then DO NOT transport the vaccines.
• If you cannot ensure vaccines are transported under proper conditions to maintain the cold
chain, then DO NOT transport the vaccines.
Vaccine Transportation Standard Operating Procedures
1. Vaccines are attended at all times during transport.
2. Vaccines are never placed in the trunk of a vehicle.
3. Vaccines are delivered directly to the facility.
4. Receiving facility promptly unpacks and appropriately stores vaccines.
5. Use a calibrated temperature monitoring device with continuous monitoring and recording
capabilities during transport.
Varicella-Containing Vaccines
The vaccine manufacturer does not recommend transporting varicella-containing vaccines (MMRV,
VAR). If these vaccines must be transported, CDC recommends the following transportation guidelines.
• Transport only in a portable freezer unit that maintains the temperature between -50°C and -15°C
(-58°F and +5°F).
Use of dry ice is not recommended for temporary storage or emergency transport. Dry ice
may subject varicella-containing vaccines to temperatures colder than -50°C (-58°F).
• The Illinois VFC program will review requests to transport varicella on a case-by-case basis to
ensure transportation guidelines are followed.

02/07/2020 Page 47 of 73
Packing Vaccines for Transport
1. Gather the supplies
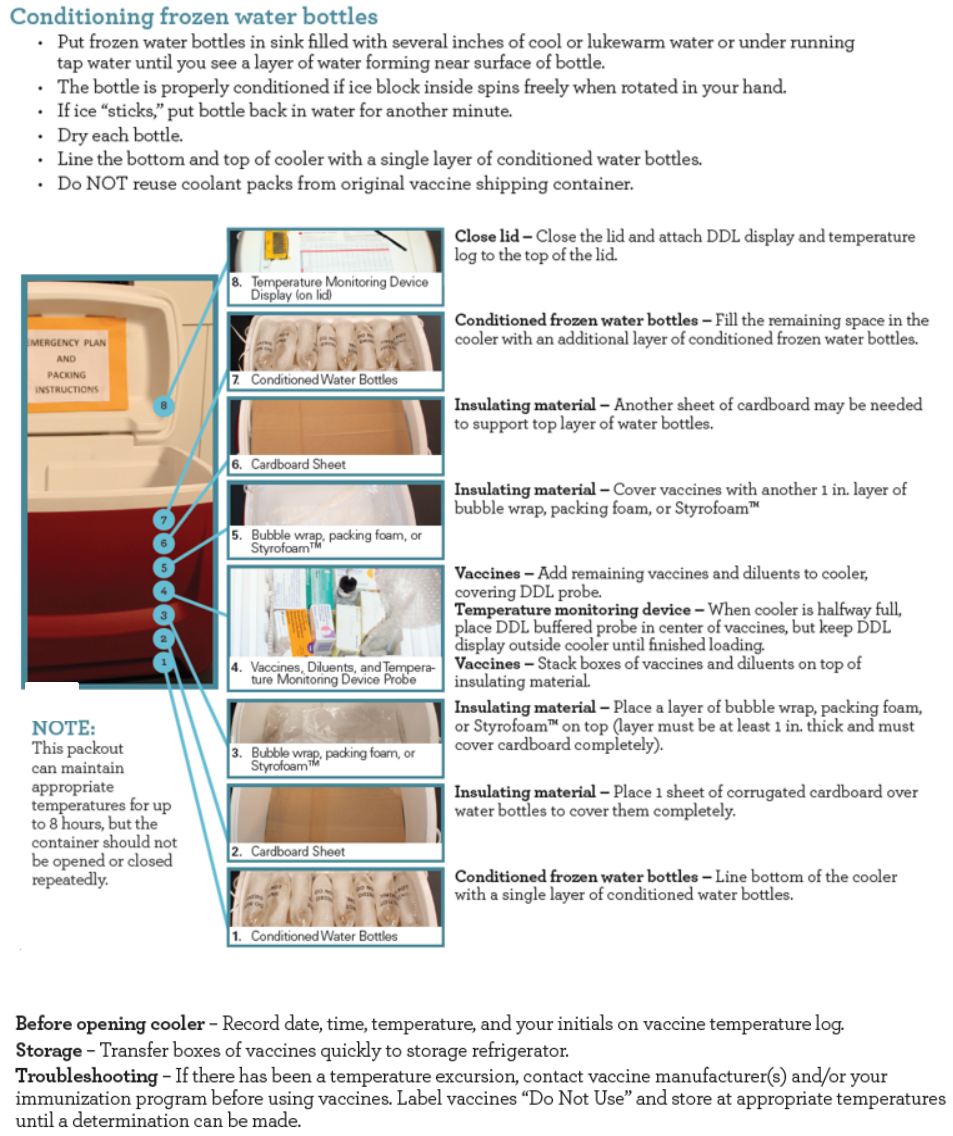
02/07/2020 Page 48 of 73
2. Pack for transport
3. Arrive at destination

02/07/2020 Page 49 of 73
TRANSPORT SYSTEM RECOMMENDATIONS
Type of Transport System
Emergency Transport/
Vaccine Transfer
Transport for Off-Site
Clinic
Portable Vaccine Refrigerator or Freezer
Yes
Yes
Qualified Container and Packout
Yes
Yes
Conditioned Water Bottle Transport System
Yes
No
Manufacturer’s Original Shipping Container
Yes (last resort only)
No
Food/Beverage Coolers
No
No
Coolant materials such as phase change materials (PCMs) may be purchased to maintain vaccines at
proper temperatures of 4° C–5° C (39° F–41° F). Follow the manufacturer’s instructions for use to reduce
the risk of freezing vaccines during transport.
Do not use frozen gel packs or coolant packs from original vaccine shipments to pack refrigerated
vaccines. They can still freeze vaccines even if they are conditioned or appear to be “sweating.”
In emergency situations, a system using conditioned water bottles can be used. Manufacturers’ original
shipping containers may also be used as a last resort in emergency situations.
MOVING TO A NEW LOCATION
VFC providers planning to move their clinic to a new location must notify the immunization program
before the clinic moves so the equipment and plan to transport the VFC vaccines may be reviewed and
approved. Contact the Illinois VFC program at [email protected] or by telephone at 217-785-
1455.
The Illinois VFC Program needs to know the steps planned to ensure that vaccine cold chain is
maintained before, during, and after the move, including the following information.
• The date of the move;
• Current address;
• New address (including suite or room numbers and zip code);
• Any changes to the clinic/organization name;
• Any change to the medical provider who signed the VFC enrollment agreement;
• Any change to the vaccine coordinators – primary and backup(s);
• Vaccine storage equipment – new equipment or moving existing equipment;
• Vaccine storage plans during the move;
• Vaccine storage plans until the storage equiment temperatures are stabilized; and
• Plans for transporting the vaccines, including frozen vaccines.
VFC ordering privileges will be suspended before the move to ensure a vaccine shipment is not
compromised. Depending upon the circumstances surrounding a move, the Illinois VFC program may
also require a site visit to be conducted before reinstating VFC ordering privileges.
Moving or installing a new refrigerator and freezer will take time to stabilize the temperatures within
the unit. It may take two to seven days to stabilize the temperature between 2 C and 8 C (36 F and 46 F)
in a newly installed or repaired refrigerator. Likewise, it may take two to three days to stabilize the
temperature between -50 C and -15 C (-58 F and +5 F) in a newly installed or repaired freezer.
VFC providers must record refrigerator and freezer temperatures a minimum of two times each
workday, including minimum/maximum temperatures one time each morning to make sure

02/07/2020 Page 50 of 73
temperatures are within appropriate ranges for at least two days before using units to store vaccines.
(Source: CDC Storage and Handling Toolkit).
EXPIRED, SPOILED, OR WASTED VACCINES
Vaccines that are expired/spoiled or wasted must be reported in I-CARE within one (1) week of the
expiration date of the vaccine. All unopened vials and manufacturer’s pre-filled syringes of spoiled or
expired vaccine received from the VFC program must be returned within six (6) months of the expiration
date for Excise Tax Credit and disposal to McKesson Specialty. Failure to report wasted vaccine to the
Illinois VFC program may result in your facility no longer being able to receive state-supplied vaccine.
VFC providers may be required to replace any excessive amounts of wasted vaccines or frequent reports
of wasted vaccines with privately-purchased vaccines.
If the vaccine(s) were exposed to temperature excursions, complete the vaccine incident report BEFORE
wasting the vaccines to determine if the suspected vaccine is viable or not.
To enter expired or wasted vaccines in I-CARE, go to the “Vaccines” page and click on “Vaccine Lots.”
• Click on the lot number to be reported as expired, spoiled, or wasted.
• Click on the “Add Transaction” button.

02/07/2020 Page 51 of 73
• Enter the required information to complete the transaction.
• Select the appropriate transaction type: Expired/Spoiled (vaccines to be returned to McKesson)
or Waste (vaccines cannot be returned to McKesson).
• Select the appropriate waste code to describe why the vaccines can no longer be administered.
• The transaction screen in I-CARE will provide guidance if additional action needs to be taken
before the vaccine may be reported as expired or wasted.
• When replacement is required, your privately purchased vaccines will replace the
wasted/expired VFC vaccines and will be entered in during the waste transaction to be added to
your VFC inventory.
The following vaccines should be returned to McKesson:
• Spoiled or expired product in its original vial or manufacturer pre-filled syringe.
• Unused manufacturer pre-filled syringes with an NDC printed on them.
The following vaccines should NOT be returned to McKesson:
• Used syringes, with or without needles
• Broken vials
• Syringe that was drawn up but not used (the VFC program discourages the use of pre-drawing
any vaccine)
• Any multi-dose vial from which some doses have been withdrawn
• IG, HBIG, or PPD
• Diluent (expired or not expired)
• Private purchased vaccine.
The vaccines not returned to McKesson must be disposed of according to usual medical biosafety
procedures, and according to your agency procedures. Federal excise tax (FET) credits can only be

02/07/2020 Page 52 of 73
processed for unopened vials and for unopened manufacturer prefilled syringes. Returns of products
other than these are not eligible for FET credit.
Providers reporting expired/spoiled vaccines will receive an e-mail when the report has been received
by CDC and should expect a return label via US mail within seven to 14 days of the e-mail date.
RETURN MAILING LABELS
A return UPS mailing label will be sent to the provider via USPS mail. The envelope containing the return
mailing label is approximately 6.75” x 4.5” and has the wording “Return Label for Expired Vaccines”
printed in red font (see the sample on the following page). The return mailing label may be addressed
generically as “Attn: VFC Vaccine Contact.” Providers may want to advise their mail room of the identity
of their primary or backup VFC vaccine contact so the mailing label may be forwarded to the correct
person.
Return mailing labels are only valid for 30 days. If the return label has not been used within 30 days,
please contact us by clicking on “Contact Us” in I-CARE and select “VFC Illinois” as the category.
MULTIDOSE VIALS
Opened IPOL multidose vials can be used until the expiration date printed on the vial unless the vaccine
is contaminated or compromised. The IPOL package insert (available at
https://www.vaccineshoppe.com/image.cfm?doc_id=5984&image_type=product_pdf
) does not require
the use of a beyond use date (BUD).
The Joint Commission has specifically addressed the issue of discarding open multi-dose vaccines in the
Joint Commission Standards Frequently Asked Questions (available at
https://www.jointcommission.org/
):
Question: Do vaccines need to follow the 28-day rule?
Answer: “Currently, vaccines are exempted from this requirement. The CDC Immunization
Program states that vaccines are to be discarded per the manufacturer's expiration date. The
Joint Commission is applying this approach to all vaccines (whether a part of the CDC or state
immunization program or purchased by healthcare facilities) with the understanding that the
vaccines are stored and handled appropriately (correct temperature is maintained, frequency of
temperature checks, etc.). Following the guidelines provided in the package insert is very
important to assure integrity of the vaccine.”

02/07/2020 Page 53 of 73
The Epidemiology and Prevention of Vaccine-Preventable Diseases: The Pink Book: Course Textbook -
13th Edition (2015) (available at http://www.cdc.gov/vaccines/pubs/pinkbook/vac-storage.html
) states,
“A multidose vial of vaccine that has been stored and handled properly and is normal in appearance can
be used through the expiration date printed on the vial unless otherwise stated in the manufacturer’s
product information.”
Sanofi Pasteur has confirmed that multi-dose vials of IPOL can be used until the expiration date on the
vial unless the vaccine in contaminated or compromised. Sanofi Pasteur also states only the number of
doses indicated in the manufacturer’s package insert should be withdrawn from the vial. After the
maximum number of doses have been withdrawn, the vial should be discarded, even if there is residual
vaccine or the expiration date has not been reached. CDC advises to never use partial doses from two or
more vials to obtain a dose of vaccine. The letter from Sanofi Pasteur is shown on the following pages.

02/07/2020 Page 54 of 73

02/07/2020 Page 55 of 73

02/07/2020 Page 56 of 73
11. VACCINE MANAGEMENT PLAN
STANDARD OPERATING PROCEDURES
VFC providers must develop, maintain, and implement a Vaccine Management Plan with detailed and
up-to-date standard operating procedures for routine and emergency vaccine management.
The Illinois VFC program has created a vaccine management plan template, which is available in I-CARE
on the home page under “Immunization Links.” The responsibilities listed in the vaccine management
plan are those of the primary and backup vaccine coordinators.
A copy of the Vaccine Storage and Emergency Response Plan must be posted on all
refrigerators/freezers used to store VFC vaccines.
Office staff handling or administering vaccines should be familiar with the vaccine management plan,
which includes the vaccine storage and emergency response plan, and ensuring vaccines are maintained
within the required temperature range.
The VFC program recommends that providers use the vaccine management plan template developed by
the Illinois VFC program as it covers all required elements. Providers may create their own vaccine
management plan, but it must include all the following items.
• Name of the current primary vaccine coordinator and at least one backup coordinator
• Signature, name, and title of the person completing the plan
• Date the plan was completed
• Contact information for individuals with 24-hour access to the building
• General operations for the following vaccine storage and handling practices:
o Proper vaccine storage and handling practices
o Temperature monitoring
o Vaccine storage (e.g., equipment, placement)
o Vaccine shipping and receiving procedures
o Vaccine ordering procedures
o Inventory control (e.g., stock rotation)
o Vaccine expiration, spoilage, and wastage prevention (e.g., protocol for responding to and
reporting vaccine loss)
o Protocols for vaccine storage equipment maintenance
o Protocols for the correct placement of vaccines within storage units
o Protocols for responding to vaccine storage and handling problems
• Staffing
o Descriptions of the roles and responsibilities of the primary and alternate (backup) vaccine
coordinators
o Policy on education and training for facility staff
o Staff training and documentation of training on VFC requirements, including proper vaccine
storage and handling
• Emergency response plan:
o The emergency response plan must include guidance on what to do in the event of
refrigerator or freezer malfunctions, power failure to vaccine storage units, natural
disasters, or other emergencies that might compromise appropriate vaccine storage
conditions.
o Contact information for emergency storage locations.
o Contact information for refrigerator and freezer maintenance and repair companies.

02/07/2020 Page 57 of 73
o Contact information for the vaccine storage unit alarm company (if applicable).
o Sources for packing materials, calibrated temperature monitoring devices, and portable
refrigerator/freezer units or qualified containers.
o In addition, the plan must include policies and protocols for maintaining the vaccine cold
chain during transport to and while stored in emergency storage locations.
EMERGENCY RESPONSE
• An on-site generator can prevent having to transport vaccines to an alternative storage facility
during a power outage. A backup battery power source can also be used in lieu of a generator.
• Backup generators or battery power sources should be tested quarterly and serviced annually
(check the manufacturer’s guidance for testing procedures and maintenance schedules).
(Source: CDC Storage and Handling Toolkit).

02/07/2020 Page 58 of 73
12. VFC SITE VISITS
To ensure the quality of VFC vaccine and the integrity of the VFC program, the Illinois VFC program
conducts the following type of provider site visits.
• Enrollment
• Compliance
• Storage and handling
• Educational
VFC visits help determine compliance with VFC program requirements. This includes identifying
potential issues with VFC vaccine accountability and determining whether VFC vaccines are being
handled, stored, and administered in accordance with the laws and policies governing the VFC program.
The review and evaluation of VFC provider practices involves assessing verbal, written, and visual
evidence encountered during the visit to determine if provider sites are following the requirements of
the VFC program.
The goals of these visits are to:
• Identify areas where providers are doing well and areas needing additional follow-up.
• Identify the educational needs of VFC providers to support meeting program requirements.
• Ensure that VFC-eligible children receive properly managed and viable vaccine.
Additionally, site visits are critical opportunities to engage provider staff and develop and strengthen
ongoing relationships.
As defined in the VFC enrollment agreement, VFC providers agree to participate in VFC program
compliance site visits including unannounced visits, and other educational opportunities associated with
VFC program requirements.
VFC compliance staff finding or observing storage and handling practices that compromise the safety
and efficacy of the VFC vaccines have the authority to act on behalf of the Illinois VFC program to
retrieve and remove the VFC vaccines from the provider. Replacement may be required under the VFC
Vaccine Loss and Replacement policy.
VFC COMPLIANCE VISIT
All enrolled and active VFC providers must receive a VFC compliance site visit every 24 months, at
minimum, to ensure compliance with VFC program standards.
• Enrolled and active providers are providers that are enrolled in the VFC program and have
ordered vaccine within the past 12 months.
• Conducting a VFC compliance site visit with providers every 24 months is a minimum-level
requirement. Providers may receive a VFC compliance site visit on a more frequent basis.
• A new provider must be enrolled and active in the VFC program at least three to six months
before receiving a VFC compliance site visit.
The VFC compliance visit requires availability of key staff that can accurately provide a realistic picture of
how the clinic is implementing the VFC program on a daily basis. The VFC compliance site visit includes
staff guidance and education on “best practices” to store and manage VFC vaccines, ensure all VFC-
eligible children are receiving properly maintained vaccines, and address practice-based questions about
VFC program initiatives.
02/07/2020 Page 59 of 73
STORAGE AND HANDLING SITE VISIT
The vaccine storage and handling visit serves as a “spot check” for proper practices on storage and
handling of VFC vaccine. The goal of these visits is to provide guidance and education, to protect the
vaccine, and to ensure VFC-eligible children are receiving properly managed vaccines.
VFC providers may be prioritized for an unannounced storage and handling visit based on the following:
• The provider’s previous history with storage and handling compliance issues;
• Time since the last site visit;
• A newly enrolled provider; or
• Providers having excessive or habitual waste in the previous 12 months. Excessive waste is
defined as wasted vaccine amounts that either exceeds $1,500 in value or three (3) percent of
the total amount of vaccines received in the previous 12 months.
The CDC Vaccine Storage and Handling Toolkit outlines is available at
https://www.cdc.gov/vaccines/hcp/admin/storage/toolkit/storage-handling-toolkit.pdf
. The toolkit
outlines best practice strategies and recommendations on the following:
• Vaccine cold chain
• Storage and handling plans
• Staff
• Vaccine storage equipment
• Temperature monitoring equipment
• Vaccine storage and handling best practices
• Storage unit temperature monitoring
• Troubleshooting
• Vaccine inventory management
• Vaccine deliveries
• Vaccine transport
• Vaccine preparation
• Vaccine disposal
Please be advised that checks to monitor vaccine storage unit temperatures by pharmaceutical
representatives or other entities do not satisfy the CDC mandate for storage and handling visit
requirements.
CONDUCTING THE SITE VISIT
The VFC site visits are conducted either by the Illinois Department of Public Health’s immunization staff
or by local health departments trained by the Illinois VFC program to act as delegates to perform
compliance visits.
FOLLOWING UP AFTER THE SITE VISIT
During or at the end of the VFC compliance site visit, VFC staff shall provide education to the provider
staff when non-compliant behaviors or practices are observed or encountered to correct the situations.
If the provider is found to be non-compliant, a provider follow-up plan will be completed and reviewed
with the provider office.

02/07/2020 Page 60 of 73
13. VACCINE LOSS AND REPLACEMENT
Vaccine accountability is a cornerstone of the VFC program and one of the program’s highest priorities.
Vaccine losses are absorbed directly by the VFC program’s budget. Since the Illinois VFC program is so
important to the health and well-being of the children in Illinois, it is essential that every dose of vaccine
is used to provide protection against preventable diseases. All VFC providers should continually monitor
vaccine storage and handling practices. Providers may contact the Illinois VFC program to request an
educational visit regarding vaccine storage and handling.
The Vaccine Loss and Replacement policy serves as the Illinois VFC program’s policy for management of
incidents that result in loss of vaccines provided through the VFC program, including VFC, 317, CHIP, or
other state purchased vaccine (hereafter referred to as “VFC program vaccines”). VFC providers are
required to report all wasted, expired, spoiled or lost vaccine to the Illinois VFC program.
Dose-for-dose replacement with privately purchased vaccine for VFC program vaccine may be required
and provider’s ordering privileges may be suspended until replacement is made. Providers having
excessive or habitual waste in the previous 12 months may also receive a storage and handling visit.
Excessive waste is defined as wasted vaccine amounts that either exceeds $1,500 in value or three (3)
percent of the total amount of vaccines received in the previous 12 months.
DEFINITIONS
Wasted: Any vaccine that cannot be used.
Expired: Any vaccine with an expiration date that has passed.
Spoiled: Any vaccine that exceeds the limits of the approved cold chain procedures or is pre-drawn and
not used within acceptable time frames. Always consult with the vaccine manufacturer and Illinois VFC
program before determining that the vaccine is spoiled or non-viable.
Lost: Vaccines that a commercial carrier (FedEx or UPS) does not deliver or does not deliver in a timely
manner. This includes VFC vaccines the provider cannot locate, account for, thrown away, or disposed
of against VFC policies.
SITUATIONS REQUIRING VACCINE REPLACEMENT
Below is a list of situations that require dose-for-dose replacement with privately-purchased vaccines.
EXPIRED VACCINE
• Failure to rotate or attempt to transfer vaccine that results in expired vaccine. VFC providers
should document transfer attempts on the “VFC Vaccine Transfer Contact Log”.
• Provider orders of vaccines that exceed the provider profile on file which results in excessive
expired inventory.
SPOILED VACCINE
• Pre-drawn vaccine that is not used. The Illinois VFC program strongly discourages the practice of
pre-drawing vaccine.
• Handling and storage mishaps by provider staff.
• Vaccine that is left out of the refrigerator or freezer and becomes non-viable. Call the vaccine
manufacturer first to help you determine the stability/viability of vaccine left out of the
refrigerator/freezer and complete the VFC Vaccine Incident Report.
• Vaccine stored in dorm style refrigerators or household combination refrigerator/freezer unit.

02/07/2020 Page 61 of 73
• Freezing vaccine that is supposed to be refrigerated.
• Refrigerating vaccine that is supposed to be frozen.
• Refrigerator/freezer left unplugged.
• Refrigerator/freezer door left open or ajar.
• Refrigerator/freezer equipment problems where proof of repair or equipment replacement is
not provided to the Illinois VFC program within 30 days from the date you became aware of the
situation.
• Power outages in which the provider fails to follow the facility’s Vaccine Storage and Emergency
Response Plan.
• Vaccine that is considered spoiled due to the provider not checking, reviewing, and
documenting refrigerator and freezer temperatures twice daily for a minimum of three days a
week.
• Vaccine that is considered spoiled due to the provider failing to use currently certified calibrated
data loggers (as primary and backup data loggers) in each VFC storage unit to check
temperatures twice daily.
• Vaccine that is spoiled and must be wasted because a provider did not take immediate or
appropriate action on out-of-range temperatures to prevent vaccine from becoming spoiled.
• Provider not available to receive a delivery of vaccines during provider’s posted hours on file
with the order and vaccine was exposed to temperature excursions during return to McKesson.
WASTED VACCINE
• VFC program vaccines given to children or adults who are not eligible to receive it based on the
most recent VFC eligibility criteria and Illinois immunization guidelines.
• VFC program vaccines administered to children or adults with the State’s Children Health
Insurance Program (CHIP) coverage (Medicaid Title XXI [21] or State-funded).
• Discarding vaccine before the manufacturer’s expiration date (includes multi-dose vials
discarded after 30 days).
• Excessive waste that either exceeds $1,500 in value or three (3) percent of the total amount of
vaccines received in the previous 12 months.
LOST VACCINES
• VFC vaccines the provider cannot locate, account for, may have been thrown away, or disposed
of against VFC policies.
OTHER
• Failure to call the VFC program within two (2) hours of receiving a VFC delivery when the
delivered vaccines do not match the packing list or I-CARE inventory.
• Failure to call the VFC program within two (2) hours to report damaged or compromised VFC
vaccine delivery.
• Transferring or transporting VFC vaccines, either refrigerated or frozen vaccines, to another VFC
provider without IDPH pre-approval.
• Transferring or transporting varicella-containing vaccines to another VFC provider without IDPH
approval on the transportation unit.
• Transferring or distributing VFC program vaccines to any non-VFC provider (also referred to as
“depoting” vaccines).

02/07/2020 Page 62 of 73
SITUATIONS NOT REQUIRING VACCINE REPLACEMENT
Below is a list of situations that are NOT considered “provider negligence.” This list is not exhaustive. In
these situations, the provider is deemed not to be at fault. You may be required to produce a letter from
the alarm/alert company or the power company.
• A commercial carrier or USPS does not deliver to the provider in a timely manner and the
provider was available to receive the vaccine during provider’s posted hours. Before making the
determination that the vaccine is non-viable, first call the vaccine manufacturer.
• A provider who has a contract with an alert/alarm company has a refrigerator that malfunctions,
and the alarm/alert company does not notify the provider.
• A provider moves vaccine to a nearby hospital due to anticipated inclement weather, the
hospital experiences a power failure, and the Illinois VFC program later deems the vaccine not
viable.
• Power was interrupted or discontinued due to a storm or act of nature, and the provider can
confirm that the facility’s Vaccine Storage and Emergency Response Plan was followed and after
consultation with the vaccine manufacturer(s) and the Illinois VFC program, it is determined that
vaccine is not viable.
• A vial that is accidentally dropped or broken by a provider.
• Vaccine that is drawn after physician orders and parental agreement during the visit, but not
administered due to parental refusal or a change in physician orders.
• Expired vaccine that is not due to provider negligence (including seasonal influenza vaccine).
• Extraordinary situations not listed above which are deemed by Illinois VFC program to be
beyond the provider’s control.
• Refrigerator/freezer equipment problems where proof of repair or equipment replacement is
provided to the Illinois VFC program within 30 days from the date you became aware of the
situation.
PROCEDURES FOR VACCINE REPLACEMENT
This updated policy applies to any VFC vaccine documented as wasted.
• The provider will receive a notice from the Illinois VFC program or will be instructed via I-CARE
that replacement of VFC vaccines with privately purchased vaccines is required.
• If proof of replacement is required, acceptable proof is packing list or paid invoice showing type,
amount, lot number and expiration date of privately purchased vaccine that will then be marked
and used as VFC vaccine.
• The provider must enter the privately purchased vaccine in I-CARE and record it as payback to
VFC. Guidance will be provided on how to enter the transactions in I-CARE.
• Replacement of the vaccine is due within 30 days of receiving the Illinois VFC program notice.
• The Illinois VFC program will not supply vaccine to the negligent provider until restitution has
been made. Enrollment or re-enrollment in the VFC program will not be accepted until full
restitution is made.
• If vaccine replacement is required, the VFC provider will be notified by the IDPH VFC program
staff.
ADDITIONAL INFORMATION
• Replacement vaccine: health care providers who must re-vaccinate due to negligence in failure
to keep vaccine viable (temperatures out of acceptable range) or improper administration will

02/07/2020 Page 63 of 73
be responsible for replacement of the vaccine needed to re-vaccinate. The Illinois VFC program
may inform the clinic’s vaccine coordinator of patients that need revaccination discussed with
their medical director. If revaccination is decided upon by the VFC provider’s medical director,
the Illinois VFC program may require a copy of the letter sent out to the patients advising of the
revaccination recommendation.
• Depending on the outcome of any suspected fraud investigation by Centers for Medicare and
Medicaid Services (CMS), Medicaid Integrity Group (MIG) Field Office and/or the appropriate
state Medicaid agency, providers may be required to, among other things, return all VFC
program vaccine, reimburse the state for the cost of used vaccines, and/or potentially be
terminated from the VFC program. The Illinois Department of Public Health reserves any and all
rights with respect to any future action.
PROCEDURE TO APPEAL A VACCINE REPLACEMENT
Providers may appeal the decision for replacement of wasted VFC vaccines by submitting the request in
writing either via e-mail to the Illinois Department of Public Health at DPH.Vaccin[email protected]
.
Providers must include all documentation, including the vaccine incident report, any communication,
and any other documentation supporting an appeal. Providers must include their VFC PIN on all
communication.
Possible outcomes of an appeal may include the following.
1. A partial reduction in the amount of the required vaccine replacement.
2. Granting a substitution in the vaccine replacement (e.g. a multi-vaccine in place of a single
component vaccine).
3. Extension up to 90 days for vaccine replacement.
4. Waive vaccine replacement. Factors to be considered include:
a. Prior history of vaccine incidents and/or vaccine waste;
b. Provider actions to prevent vaccine incidents from occurring again;
c. Actions at the time of incident;
d. Documentation of provider actions to transfer vaccines to other providers;
e. Change in management, medical director, and providers within clinic; or
f. Extenuating circumstances.
5. If the provider is unable to use the replacement vaccines, the replacement vaccines may be
shipped to a local health department or other approved provider.
All appeal requests will be reviewed, and the provider notified of all decisions within 30 days.

02/07/2020 Page 64 of 73
14. FRAUD AND ABUSE
OVERVIEW
As childhood vaccines become more expensive and immunization programs more complex, the VFC
program becomes vulnerable to fraud and abuse. A working understanding of what constitutes fraud
and abuse is critical for all persons working in the VFC program. Consistent with “fraud” and “abuse” as
defined in the Medicaid regulations at 42 CFR § 455.2, and for the purposes of this guide, the following
definitions will be used:
Fraud: An intentional deception or misrepresentation made by a person with the knowledge that the
deception could result in some unauthorized benefit to himself or some other person. It includes any act
that constitutes fraud under applicable federal or state law.
Abuse: Provider practices inconsistent with sound fiscal, business or medical practices and result in an
unnecessary cost to the Medicaid program (and/or including actions that result in an unnecessary cost
to the immunization program, a health insurance company or patient); or in reimbursement for services
not medically necessary or fail to meet professionally recognized standards for health care. Abuse also
includes recipient practices that result in unnecessary cost to the Medicaid program.
The following are additional definitions used in the VFC program.
Oversight: Illinois specifies any suspected case of fraud and abuse should immediately be reported to
the Department’s VFC administrator, coverage level administrator, or immunization section chief.
Within five working days, the Department’s Immunization Program will contact the provider in question
or the person reporting the suspected fraud and abuse to perform an in-depth interview, with
documentation recorded on the Department’s fraud and abuse form. A file will be established for each
provider suspected of fraud and abuse with a copy of all verbal and written correspondence maintained,
as well as maintaining a fraud and abuse referral database. The Department’s Immunization Program
will follow-up with the external agency within ten working days, or sooner.
Enforcement: If the VFC program determines from the assessment of information available that the
situation requires referral for further investigation by an outside agency, the VFC program will make
these referrals within ten working days from assessment. All suspected cases of fraud and abuse that
require further investigation must be referred first to the immunization section chief or equivalent for
referral to the Medicaid Integrity Group (MIG) and the CDC, with notification of the referral also sent to
Department’s legal counsel and auditor.
Termination: The Department’s Immunization Program has the right to exclude or terminate providers
from the VFC program that are not following any Illinois VFC program or I-CARE requirements. Vaccine
will be removed from the provider’s possession and the provider will be prohibited from receiving future
shipments until the exclusion is lifted. The terminated provider or entity may be eligible to re-apply for
the VFC and I-CARE Programs after the exclusion is lifted. The Illinois VFC program will terminate
providers from participating in the VFC program if the provider is found to be in non-payment status
under Medicare, Medicaid, and other federal health care programs. Termination of providers may also
occur due to Office of Inspector General (OIG) sanction, failure to renew license or certification
registration, revocation of professional license or certification, or termination by the Illinois Medicaid
Agency. Providers that are terminated from the VFC program (both voluntarily and involuntarily) will be
removed from active status in the VFC program, removed from VFC State of Illinois Rapid Electronic
Notification (SIREN) lists, and excluded on reports to the Illinois Medicaid agency requesting data on
active VFC providers.

02/07/2020 Page 65 of 73
All cases of suspected fraud and abuse will be handled according to this policy and the CDC Non-
Compliance with VFC Requirements Protocol.
FRAUD AND ABUSE POLICY
The Fraud and Abuse Policy is a comprehensive written policy that addresses prevention, detection,
investigation, and resolution of fraud and abuse allegations. VFC staff must be familiar with this policy
and be able to prevent, to identify and to follow-up on situations that involve suspected fraud or abuse
of the VFC program.
When providers enroll in the VFC program, they agree to comply with all the requirements of the
program. Lack of adherence to the VFC program requirements by an enrolled provider could lead to
fraud and abuse of the VFC program by that provider.
Failure to comply with VFC requirements is defined as:
• Any VFC-enrolled provider who is identified as not maintaining any of the federal requirements
for the VFC program as defined in the enrollment agreement.
Failure to comply may be identified by:
• VFC program staff
• The enrolled provider’s staff, or
• A third party
Non-compliance with program requirements may occur due to an unintentional lack of understanding of
the VFC program requirements, or the behavior may be intentional. If the non-compliance appears
intentional and the provider has received financial benefits from the behavior, the situation would
require immediate referral to an outside agency for investigation of suspected VFC fraud and abuse.
EXAMPLES OF FRAUD AND ABUSE
Fraud or abuse can occur in many ways, and some types of fraud and abuse are easier for the VFC
program to prevent or detect than others, depending on how the VFC program is implemented. The VFC
program will use provider profiles, ordering patterns, VFC site visits, temperature logs and doses
administered reports to monitor provider compliance with VFC program requirements. Some examples
of potential fraud are:
• Providing VFC vaccine to non-VFC-eligible children
• Selling or otherwise misdirecting VFC vaccine
• Billing a patient or third party for VFC-funded vaccine
• Charging more than the established maximum regional charge ($23.87) for administration of a
VFC funded vaccine to a federally vaccine-eligible child
• Denying VFC-eligible children VFC-funded vaccine because of parents’ inability to pay for the
administration fee
• Failing to implement provider enrollment requirements of the VFC program
• Failing to screen for and document eligibility status at every visit
• Failing to maintain VFC records and comply with other requirements of the VFC program
• Failing to fully account for VFC-funded vaccine
• Failing to properly store and handle VFC vaccine
• Ordering VFC vaccine in quantities or patterns that do not match the provider’s profile or
otherwise over-ordering VFC doses of vaccine
• Waste of VFC vaccine

02/07/2020 Page 66 of 73
ALLEGATIONS OF SUSPECTED FRAUD AND ABUSE
The Department will investigate all allegations of suspected fraud and abuse and will determine if the
situation is intentional fraud and abuse or unintentional abuse or error due to an excusable lack of
knowledge of the VFC program with no purposeful intent to misrepresent or defraud the VFC program.
If the situation is found to be unintentional, an educational intervention will be made, and
arrangements will be established to replace any vaccine used inappropriately.
The Department’s Immunization Program staff will provide in-depth education to the provider’s key
staff about the VFC program and Illinois VFC enrollment and accountability requirements. The provider
will be required to complete and return an acknowledgement of receipt of the follow-up plan detailing
the steps that will be taken to prevent further incidents. This signed plan must be returned within one
month. The provider will be advised that any recurrence of suspected fraud and abuse may result in
termination from the VFC program and referral to an external agency for investigation.
If the investigation determines the situation is intentional, the situation will be reported to an external
agency for investigation. All suspected cases of fraud and abuse that require further investigation will
first be referred to the immunization section chief or equivalent for review by the Office of Health
Protection and the Department’s legal counsel and auditor. Suspected cases of fraud and abuse will
then be referred to the Medicaid Integrity Group (MIG) and the CDC.
Suspected cases of fraud and abuse will be referred to the Centers for Medicare and Medicaid Services
(CMS), Medicaid Integrity Group (MIG) Field Office for further investigation. CMS/MIG may refer the
suspected case to the appropriate state Medicaid agency for further investigation. VFC ordering
Failure to
Comply with
VFC
Requirements
Mishandling of VFC Vaccine
•Improper storage and handling
•Lost, wasted/expired vaccine
Billing and Office Practices
•Billing for VFC vaccine
•Charging above $23.87 for
administration fee
•Failure to maintain VFC records for
3 years
•Failure to comply with VFC
ordering or site visit requirements
Misuse of VFC Vaccine
•Refusing to provide VFC vaccine
due to inability to pay
administration fee
•Using VFC vaccine on non-VFC-
eligible children (failure to screen)
•Transfer of VFC vaccine without
program approval
•Routine borrowing of VFC vaccine
for use on non-VFC-eligible
patients
•No privately-purchased vaccine
but provider cares for insured
children

02/07/2020 Page 67 of 73
privileges may be suspended when a referral is made to CMS/MIG. Depending on the outcome of any
investigation by CMS/MIG and/or the appropriate state Medicaid agency, providers may be required to,
among other things, return all VFC vaccine, reimburse the state for the cost of used vaccines, and/or
potentially be terminated from the VFC program. The Department reserves any and all rights with
respect to any future action.
FRAUD AND ABUSE CONTACTS
Suspected VFC fraud or abuse may be reported to any of the following Department staff.
• Linda Kasebier, VFC Administrator, is designated as the primary contact.
• Karen Pendergrass, Immunization Coverage Level Administrator, is designated as first backup.
• Gina Lathan, Immunization Section Chief, is designated as second backup.
ONGOING PROVIDER MONITORING PROCEDURES
The Illinois VFC program will exclude providers from participating in the VFC program if the provider is
found to be in non-payment status under Medicare, Medicaid, and other Federal health care programs.
Exclusion of providers also may occur due to Office of Inspector General (OIG) sanction, failure to renew
license or certification registration, revocation of professional license or certification, or termination by
the state Medicaid agency. The Illinois Immunization Program will monitor OIG exclusions by checking
the List of Excluded Individuals and Entities on the OIG website (at
https://oig.hhs.gov/exclusions/index.asp
) upon provider enrollment and on a regular basis thereafter.
Providers are strongly encouraged to check the OIG website list of excluded individuals/entities prior
to hiring or contracting with any individuals or entities. Enrolled providers who employ a person
(including, but not limited to, physicians, mid-level practitioners, nurses or nursing aides) from the
excluded provider list will be terminated from the program and the state Medicaid agencies will be
notified.
The Department’s Immunization Program also has the right to exclude providers not following any other
Illinois VFC program or I-CARE requirements. Vaccine will be removed from the provider’s possession
and the provider will be prohibited from receiving future shipments until the exclusion is lifted. The
excluded provider or entity will be required to re-apply for the VFC and I-CARE Programs after the
exclusion is lifted. The Illinois Immunization Program may share information with the state attorney’s
office, and the Medicaid Fraud and Abuse Unit regarding allegations and exclusions due to fraud and
abuse.
REPORTING VFC PROVIDER TERMINATIONS
Providers terminated from the VFC program (both voluntarily and involuntarily) will be removed from
active status in the VFC program, removed from State of Illinois Rapid Electronic Notification System
(SIREN) lists and excluded on reports to the state Medicaid agency requesting data on active VFC
providers.

02/07/2020 Page 68 of 73
APPENDICES
The following documents are in the appendix.
• VFC Eligibility Status Codes
• VFC Tip Sheets
• Glossary of Important VFC Terms

02/07/2020 Page 69 of 73
VFC ELIGIBILITY STATUS CODES
The Centers for Disease Control and Prevention has updated the VFC eligibility codes. The complete list
of codes is shown in the following table.
Code
Label
Definition
V01
Not VFC eligible
Client does not qualify for VFC because they do not have one
of the statuses below. (V02-V05)
V02
VFC eligible –
Medicaid/Medicaid Managed
Care
All the following are true:
• Client is currently eligible for Medicaid or Medicaid
managed care Title XIX (19)
• Client is < 19 years old
• The type of vaccine administered is eligible for VFC
funding
V03
VFC eligible – Uninsured
All the following are true:
• Client does not have health insurance
• Client is < 19 years old
• The type of vaccine administered is eligible for VFC
funding
V04
VFC eligible – American
Indian/Alaska Native
All the following are true:
• Client is a member of a federally recognized tribe
• Client is < 19 years old
• The type of vaccine administered is eligible for VFC
funding
V05
VFC eligible – underinsured at
FQHC/RHC/deputized provider
All the following are true:
• Client has insurance, but insurance does not cover
vaccines, limits the vaccines covered or caps vaccine
coverage at a certain amount
• Client is receiving care at an FQHC, RHC or deputized
provider
• Client is < 19 years old
• The type of vaccine administered is eligible for VFC
funding
V22
CHIP
Client is eligible for the CHIP program (Medicaid Title XXI [21]
or State-funded), a separate state health insurance that is
NOT a Medicaid expansion program. The patient must
receive CHIP vaccines.
V23
317
Client is eligible to receive vaccines under the state/program
immunization policy and the vaccine administered is eligible
for 317 funding. This should only be used upon direction by
IDPH.
V24
Medicare
Client is enrolled in Medicare. The patient is not eligible for
VFC or 317 funded vaccines.
V25
State program eligibility
Client is eligible for a state vaccine program. The patient is
not eligible for VFC or 317 funded vaccines.

02/07/2020 Page 70 of 73
VFC TIP SHEETS
The Illinois VFC program has created tip sheets to assist providers with specific tasks. The following are
some of the tip sheets available in I-CARE on the home page under the “Immunization Links” tab.
• Data loggers: A tip sheet that provides a description of a data logger, explains how to identify if
your unit is a data logger, and how to review and interpret the data logger's data and reports.
• Moving to a new location: A tip sheet for moving a VFC provider clinic to a new location.
• VIS documentation: A tip sheet detailing the Vaccine Information Statement documentation
requirements.

02/07/2020 Page 71 of 73
GLOSSARY OF IMPORTANT VFC TERMS
Abuse (related to Fraud)
Provider practices that are inconsistent with sound fiscal, business, or medical practices and result in an
unnecessary cost to the Medicaid program (also includes actions that result in an unnecessary cost to
the immunization program, a health insurance company, or a patient), or in reimbursement for services
that are not medically necessary or that fail to meet professionally recognized standards for health care.
Also includes program recipient practices that result in unnecessary cost to the Medicaid program.
Advisory Committee on Immunization Practices (ACIP)
The ACIP consists of 15 medical and public health experts selected by the Department of Health and
Human Services Secretary to provide advice and guidance to the Secretary, Assistant Secretary for
Health, and CDC on the control of vaccine-preventable diseases. The committee develops
recommendations for the routine administration of vaccines to children and adults in the civilian
population, including guidance on age for vaccine administration, number of doses and dosing intervals,
and precautions and contraindications. See VFC-ACIP resolutions.
Affordable Care Act
The comprehensive health care reform law enacted in March 2010 (sometimes known as ACA, PPACA,
or “Obamacare”).
The law has three primary goals:
1. Make affordable health insurance available to more people. The law provides consumers with
subsidies (“premium tax credits”) that lower costs for households with incomes between 100%
and 400% of the federal poverty level.
2. Expand the Medicaid program to cover all adults with income below 138% of the federal poverty
level. (Not all states have expanded their Medicaid programs.)
3. Support innovative medical care delivery methods designed to lower the costs of health care
generally.
American Indian or Alaska Native (AI/AN)
As defined by the Indian Health Care Improvement Act (25 U.S.C. 1603):
• “Indians” or “Indian,” unless otherwise designated, means any person who is a member of an
Indian tribe, as defined in subsection (d) of this section, except that, for the purpose of sections
1612 and 1613 of this title, such terms shall mean any individual who (1) irrespective of whether
he or she lives on or near a reservation, is a member of a tribe, band, or other organized group
of Indians, including those tribes, bands, or groups terminated since 1940 and those recognized
now or in the future by the State in which they reside, or who is a descendant, in the first or
second degree, of any such member, or (2) is an Eskimo or Aleut or other Alaska Native, or (3) is
considered by the Secretary of the Interior to be an Indian for any purpose, or (4) is determined
to be an Indian under regulations promulgated by the Secretary.
• (d) “Indian tribe” means any Indian tribe, band, nation, or other organized group or community,
including any Alaska Native village or group or regional or village corporation as defined in or
established pursuant to the Alaska Native Claims Settlement Act (85 Stat. 688) [43 U.S.C. 1601 et
seq.], which is recognized as eligible for the special programs and services provided by the
United States to Indians because of their status as Indians.

02/07/2020 Page 72 of 73
Deputization Agreement
A formal agreement through a Memorandum of Understanding (MOU), whereby Federally Qualified
Health Centers (FQHCs) or Rural Health Clinics (RHCs) delegate their VFC authority for vaccinating
underinsured children to local health departments (LHDs), who then vaccinate underinsured children as
agents of the FQHC/RHC. For more information on deputization agreements, please contact the Illinois
VFC program at DPH.Vaccines@illinois.gov
or 217-785-1455.
Department of Health and Human Services, Office of Inspector General (OIG)
Office mandated to protect the integrity of Department of Health and Human Services (HHS) programs
and their beneficiaries. It is generally responsible for identifying, communicating and correcting activities
of waste, fraud or abuse within DHHS programs. The OIG maintains the List of Excluded Individuals and
Entities (LEIE).
Expiration Date
The last date on which the vaccine may be used; expired vaccine includes vaccine that is past the
manufacturer expiration date on the vial or expiration date after reconstitution, depending on the
vaccine and according to manufacturer instructions.
Fraud (related to Abuse)
An intentional deception or misrepresentation made by a person with the knowledge that the deception
could result in some unauthorized benefit to himself or some other person. It includes any act that
constitutes fraud under applicable federal or state law.
Health Care Sharing Ministries (HCSMs)
Nonprofit alternatives to purchasing health insurance from private, for-profit insurers. Generally, HCSMs
are organizations whose members share a common belief system and collectively “share” the cost of
their members’ medical care.
Insurance
For the purpose of the VFC program, “insurance” is defined as a plan that is:
• Regulated by a State’s Insurance Commissioner and/or
• Subject to the Employee Retirement Income Security Act of 1974 (ERISA). ERISA is a federal law
that sets minimum standards for most voluntarily established pension and health plans in
private industry to provide protection for individuals in these plans.
List of Excluded Individuals and Entities (LEIE)
Providers on the LEIE are excluded from participating in federally funded health care programs because
of issues that include program-related fraud, patient abuse, licensing board actions, and default on
Health Education Assistance Loans. This list is maintained by the OIG of DHHS.
Office of Management & Budget (OMB)
Office that assists the President in overseeing the preparation of the federal budget and supervising its
administration in Executive Branch agencies. OMB evaluates the effectiveness of agency programs,
policies, and procedures.
02/07/2020 Page 73 of 73
Rural Health Clinic (RHC)
An RHC is a clinic located in a Health Professional Shortage Area, a Medically Underserved Area, or a
Governor-Designated Shortage Area. RHCs are required to be staffed by physician assistants, nurse
practitioners, or certified nurse midwives at least half of the time that the clinic is open.
Vaccine Administration Fee
The amount a VFC-enrolled provider can charge a non-Medicaid VFC-eligible child for each vaccine
administered (also known as the administration fee or “admin fee”). State Medicaid agencies have the
authority to reimburse at a lower level than the set vaccine administration fee. The Centers for
Medicare and Medicaid Services (CMS) set and adjust these maximum regional charges.
VFC-ACIP Resolutions
The Advisory Committee on Immunization Practices (ACIP) has unique legal authority from Congress to
provide recommendations for the VFC program. When recommending a new vaccine or a change in
vaccine use, ACIP votes on a resolution to include the vaccine change in the VFC program. VFC
resolutions passed by ACIP form the basis for VFC program policies on vaccine availability and use.
Vaccines procured through the VFC program must be administered according to the guidelines outlined
by ACIP in VFC resolutions. (VFC vaccines may also be administered in accordance with state school
attendance laws.) CDC establishes contracts for VFC vaccines only after a VFC resolution is in place.
VFC-Program Eligibility Categories
• VFC-eligible child
A child who is 18 years of age or younger and meets one or more of the following criteria:
o American Indian (AI) or Alaska Native (AN)
o Medicaid-eligible/enrolled (Title XIX [19] only)
o Uninsured
o Underinsured (has health insurance, but the coverage does not include any ACIP-
recommended vaccines or includes only selected ACIP-recommended vaccines)
• Uninsured
A child who has no health insurance coverage.
• Underinsured
A child who has health insurance, but whose coverage does not include any ACIP-recommended
vaccines or only includes selected ACIP-recommended vaccines. An underinsured child is VFC-
eligible only for the vaccines that are not covered. A child whose insurance covers vaccines but
has a fixed dollar limit or cap for vaccines. Once that fixed dollar amount is reached, a child is
then eligible. Underinsured children are eligible to receive VFC vaccine only through a federally
qualified health center (FQHC), a rural health clinic (RHC), or under an approved deputization
agreement.
• Fully insured (not eligible)
A child with insurance that covers the cost of vaccine, even if the insurance plan has a high
deductible or copay, or if a claim for the cost of the vaccine and its administration would be
denied for payment by the insurance carrier because the plan’s deductible has not been met.
This child is not eligible for the VFC program.
