
1
Department of Land and
Natural Resources,
Division of Aquatic Resources
University of Hawai‘i,
Social Science Research Institute
PREPARED FOR PREPARED BY
MARCH
2017
Identifying Management Responses to
Promote Coral Recovery in Hawai‘i
Coral Bleaching
Recovery Plan
2
This report was made possible by:
The NOAA Coral Reef Conservation Program and the
Department of Land and Natural Resources, Division of Aquatic Resources
NA15NOS4820037
Cover image: Molokini crater at the peak of the coral bleaching event, October 31, 2015.
Photo credit: Darla White, Maui Division of Aquatic Resources
3
Executive Summary ............................................................................................................ 4
Section One: Introduction ................................................................................................... 6
Coral Bleaching Recovery Steering Committee ........................................................... 6
Goals and Background .............................................................................................. 6
Hawai‘i’s Mass Bleaching Event (2014/2015) ............................................................. 7
Section Two: Developing a Coral Bleaching Recovery Strategy ........................................... 12
Part 1: Defining the Role of Resource Managers ...................................................... 13
Part 2: Selecting Priority Areas ................................................................................. 17
Part 3: Gathering Expert Opinion of Ecologically Effective Management Actions ........ 18
Global Scientist Expert Judgment ...................................................................... 18
Hawai‘i-based Scientist and Manager Expert Opinion ......................................... 23
Part 4: Critically Analyzing the Effectiveness of Top-Ranked Management Actions .... 25
Section Three: Conclusions .............................................................................................. 30
Comparing results from expert judgment and the literature analysis .......................... 30
Limitations of this analysis ....................................................................................... 31
Management implications and next steps ................................................................. 31
Appendix . ..... .................................................................................................................. 32
Literature Cited ................................................................................................................ 40
Contents
.
4
GOALS / OBJECTIVES
The goal of the Coral Bleaching Recovery Plan is to promote coral reef recovery following
the 2014-2015 global coral bleaching event. Coral bleaching is a stress response, generally
induced by high temperature and light levels, where the coral animal expels zooxanthellae,
or photosynthetic dinoflagellates that provide coral polyps with energy. Bleached corals are
in a weakened state and will eventually die if temperature and light levels remain high. We
sought to identify management interventions most likely to promote coral recovery following
the mass bleaching event in Hawai‘i, specifically by synthesizing published information and
expert opinions relevant to future policy and rule making by the Department of Land and
Natural Resources (DLNR). The Coral Bleaching Recovery Plan summarizes these findings,
with the goal of supporting effective capacity to implement management actions to promote
coral recovery in Hawai‘i.
HAWAI‘I’S MASS BLEACHING EVENT (2014/2015)
In August 2014, thermal stress began to cause bleaching throughout the Hawaiian
Archipelago. In the main Hawaiian Islands, the majority of bleaching was observed around
Kauai, Oahu, and Maui [23]. In 2015, bleaching was severe, with the most extreme bleaching
occurring in west Hawai‘i and Maui. The bleaching event resulted in extensive coral mortality,
especially in west Hawai‘i and Maui. Although mortality varied among sites, overall average
coral cover loss at surveyed sites in west Hawai‘i was 49.7% as a result of the 2015 bleaching
event [25]. Bleaching mortality rates were especially catastrophic for important reef-building
species; for example, Porites lobata mortality was 55%, while for P. compressa it was 33%
[25]. Coral mortality rate of Maui’s corals was estimated at 20-40% following the 2015
bleaching event [27].
DEVELOPING A CORAL BLEACHING RECOVERY STRATEGY
To develop a strategy to promote coral recovery following the mass bleaching event, we
synthesized management recommendations in four major steps: 1) define the role of resource
managers, 2) collect global expert opinion on ecologically effective management actions,
3) collect Hawai‘i-based expert opinion on effective management actions, and 4) analyze
empirical evidence describing how the top ranked management actions could meet our
recovery objectives.
Executive Summary
5
CONCLUSIONS
Establishing a network of permanent no-take Marine
Protected Areas (MPAs) and establishing a network of
Herbivore Fishery Management Areas (HFMAs) were
the top ranked actions arising from the expert judgment
assessments and the literature analysis.
Thus, our analysis indicates that spatial management and particularly, herbivore manage-
ment, will be critical to post-bleaching coral recovery in Hawai‘i. These were top ranked
actions in both evaluations of global and local expert judgment as well as scientific literature.
Additionally, there were some differences between management actions ranked most highly
by experts and the evidence derived from the scientific literature. For example, reducing sed
-
iment stress was ranked highly by experts but did not come out as an important action from
the literature analysis. This may be because the experts that were surveyed
in this study were
not explicitly asked to consider the feasibility of each management action. Thus, as part of
our findings, we discuss the caveats of our analysis, including the limitations associated with
the use of expert opinion to inform management decisions. Our evaluations were inherent-
ly subjective, as scientific papers tend to focus on research questions rather than feasible
management outcome.
The next step in the coral bleaching recovery planning process should be to evaluate where
the top-ranked actions including spatial management and perhaps a selection of fisheries
rules would have the greatest positive impact in terms of coral reef recovery. This is still an
open question because, as the literature emphasized, management actions will not have a
consistent effect based on the natural ecological variability among different reef areas. This
spatial prioritization should consider minimizing social cost and consider the management
feasibility of actions that are seriously being considered for implementation. Finally, an evalu-
ation of how management actions could enhance resiliency to future coral bleaching events
is needed. Bleaching events are predicted to increase in both severity and frequency and so
a proactive, resilience-based management framework should be considered to support the
ability of Hawai‘i’s reefs to resist frequent climate disturbances.

6
All elements of this plan were co-developed and reviewed through an initial scientific steering committee, and then
reviewed by the Department of Land and Natural Resources (DLNR), Division of Aquatic Resources (DAR) which
also provided input to the initial structure of the plan and the final plan content.
Section One: Introduction
Coral Bleaching Recovery Steering Committee
Anne Rosinski
University of Hawai‘i (UH),
Hawaii Coral Reef Initiative
Charles Birkeland
University of Hawai‘i (UH)
Darla White
DAR
Eric Conklin
The Nature Conservancy (TNC)
Ivor Williams
NOAA Pacific Islands Fisheries Science Center
(PIFSC)
Jamison Gove
NOAA Pacific Islands Fisheries Science Center
(PIFSC)
Kelvin Gorospe
NOAA Pacific Islands Fisheries Science Center
(PIFSC)
Tom Oliver
NOAA Pacific Islands Fisheries Science Center
(PIFSC)
William Walsh
DAR
The goal of the Coral Bleaching Recovery Plan is to
promote coral reef recovery following the 2014-2015
global coral bleaching event. Coral bleaching is a stress
response, most commonly induced by high tempera-
tures and light levels, in which the coral animal expels
zooxanthellae, the photosynthetic dinoflagellates that
provide coral polyps with much of their energy. With-
out zooxanthellae, coral becomes more susceptible to
diseases and if the stress, in this case a period of high
ocean temperatures, is sustained, coral mortality will
occur. Coral bleaching events typically occur during the
warmest time of year, in Hawai
‘
i this is between August
and October.
Coral mortality caused by frequent coral bleaching
events leads to systematic changes in the structure of
tropical ecosystems [1-6]. Mass coral bleaching events
are occurring with more severity and frequency, nega-
tively affecting coral reefs worldwide with both short and
long-term impacts [7-11]. Studies of coral bleaching in
Hawai
‘
i have mainly focused on physiological processes
including acclimation potential [12,13], mechanisms
and breakdowns in coral metabolism [14,15], and the
role of reef environmental parameters and reef morphol-
ogy on coral bleaching patterns [16]. Thus, despite the
pressing consequences of increasingly frequent coral
bleaching events, direct management interventions to
promote recovery from a bleaching event have been
extremely limited [17-20].
We sought to identify management interventions that
could promote coral recovery to Hawai
‘
i’s mass bleach-
ing by synthesizing information that could directly sup-
port future policy and rule making by the Hawai
‘
i DAR.
This process began with an announcement by DAR that
they would initiate comprehensive coral reef manage-
ment planning, prompted by the unprecedented coral
bleaching throughout the state (Figure 1). The first step
in the planning process was to synthesize peer-reviewed
literature to identify the role of resource managers in
coral bleaching recovery and to collect case studies of
previous management interventions following a mass
Goals & Background

7
bleaching event. Then, we collected opinions from global
coral bleaching experts on which management inter-
ventions they felt would be most ecologically effective in
Hawai
‘i.
Through a workshop with Hawai
‘
i-based coral experts,
potential management actions were further prioritized.
The workshop group also ranked specific actions that
DAR could take in four priority areas: west Hawai
‘
i,
Maui, K ˉane‘ohe Bay, and North Kaua
‘i
. Finally, the top-
ranked management interventions were further analyzed
in a process to investigate how well each action met our
recovery objectives. The Coral Bleaching Recovery Plan
synthesizes the information garnered from these steps
to support DAR’s decision-making process to implement
management interventions to promote coral recovery
and resiliency throughout the state. Online resources for
this plan can be found at
http://dlnr.hawaii.gov/reefresponse/.
Figure 1.
Timeline of planning process
steps from announcement of
the Coral Bleaching Recovery
Plan to public release.
Hawai‘i’s Mass Bleaching
Event (2014/2015)
2014
Beginning in early spring 2014, NOAA
Coral Reef Watch reported the appearance
of positive sea surface temperature (SST) anomalies that
suggested the development of an El Niño event [21].
The coral bleaching event was specifically triggered by a
combination of warming in the North Pacific Ocean, was
the Pacific Decadal Oscillation, and “The Blob”, a large
mass of warm ocean water that developed and stayed in
the Pacific Ocean off the coast of North America [21].
By late August 2014, thermal stress began to cause
bleaching throughout the Hawaiian Archipelago. The
high thermal stress in Hawai‘i started in the central por-
tion of the archipelago around the Northwest Hawaiian
Islands (NWHI).
The accumulation of thermal stress can be measured
in Degree Heating Weeks (DHW). For example, if sea
surface temperatures exceed the bleaching threshold
for by one degree for one week, that’s a DHW value of
1. When the DHW metric reaches 4 °C-Weeks, sub-
stantial coral bleaching typically occurs. If DHW values
reach 8 °C-Weeks, widespread bleaching is likely and
significant coral mortality can be expected. During the
2014 event in the NWHI, certain areas experienced up
to 15 °C-Weeks (see Appendix A for NOAA Coral Reef
Watch satellite data). This marked the third and most
severe coral bleaching event on record in the NWHI.
Areas severely affected included French Frigate Shoals
and Lisianski Island, especially on Montipora-dominated
reefs [22].
High temperature anomalies then spread both to the
west and east, reaching the main Hawaiian Islands
in late-September 2014 [21]. In the main Hawaiian
Islands, the majority of bleaching was observed around
Kaua‘i, O‘ahu, and Maui [23]. Areas of K ˉane‘ohe Bay,
O‘ahu were especially impacted in part because of com-
pounding effects of a flooding event during the bleach-
November 2015
January–March 2016
February–May 2016
August 2016
October 2016–February 2017
March 2017
DAR announced development of a
Coral Bleaching Recovery Plan
Collect global opinions through the
Coral Bleaching Recovery Survey
Synthesized peer-reviewed literature
related to coral bleaching and recovery
Analyzed recommendations with local
expert researchers and DAR staff at the
Coral Bleaching Recovery Workshop
Writing the Coral Bleaching
Recovery Plan
The final Coral Bleaching Recovery
Plan is released

8
ing event [24]. DAR surveys indicate that over 10 spe-
cies of coral were affected by the 2014 bleaching event
in K ˉane‘ohe Bay [23]. On average, three out of four
dominant coral species’ colonies exhibited some sign of
bleaching, with northern areas of K ˉane‘ohe showing the
worst bleaching, while reefs in the central part of the bay
exhibiting less bleaching [23].
In K ˉane‘ohe Bay, the majority of coral colonies tagged
by DAR had returning color and were recovering in
December 2014, while 12% of colonies had died
(Figure 2). Relative to O‘ahu, other areas including
west Hawai‘i and Maui had some moderate to minimal
bleaching in 2014.
Figure 2.
A coral colony tagged in
K ˉane‘ohe Bay by DAR showing
significant bleaching in October
2014 (left) and re-coloring in
December 2014 (right).
Photos: DAR
2015
Global-scale bleaching occurred again in
2015, and the NOAA Coral Reef Watch
program declared the third ever global coral bleach-
ing event based on their suite of satellite monitoring
products [25]. This intense temperature anomaly again
resulted in coral bleaching throughout the Hawaiian
archipelago, this time with higher severity particularly in
the southern islands (Figure 3).
Figure 3.
Images from coral bleaching survey sites in west Hawai‘i
a) severely bleached Porites evermanni at N. Keauhou,
b) severely bleached Pocillopora eydouxi and Porites
lobata colonies at Honokoˉhau, c) initial turf colonization
on P. evermanni at N. Keahou, d) and e) initial algal turf
colonization on P. lobata at Honokoˉhau, and f) algal turf
colonization of recently dead P. evermanni at N. Keauhou
(post-bleaching mortality), from Kramer et al. 2016.

9
The stress exhibited on corals from the 2015 event
peaked at 12 °C-Weeks. Severe mass bleaching was ob-
served in west Hawai‘i and Maui, with minimal bleaching
observed around O‘ahu and Kaua‘i. In west Hawai‘i,
there was site-level variation in bleaching prevalence,
in South Kohala region averaging 53% but other areas
in west Hawai‘i reaching up to 93% average bleaching
prevalence [26, 27]. Among the most affected sites were
shallow regions at Kanekanaka, Kawaihae and ‘O
ˉ
hai‘ula
(Spencer Beach) where 80-85% of the corals severely
bleached [26]. Bleaching was also observed on Maui,
particularly on southern and western-facing shores [28].
West Hawai‘i and Maui had the highest levels of mor-
tality following the 2015 bleaching event (Figure 4,
Figure 5). Although mortality varied among sites, overall
average coral cover loss at surveyed sites in west Hawai‘i
was 49.7% as a result of the 2015 bleaching event [26].
Bleaching mortality rates were especially catastrophic
for important reef-building species; for example, Porites
lobata mortality was 55%, while for P. compressa it was
33% [26]. Coral mortality rate of Maui’s corals
was estimated at 20-40% following the 2015 bleaching
event [28].
Figure 4. Percent change in hard coral cover between 2013/2015 and 2016 NOAA-PIFSC CREP Fish Team
visual surveys. Data and graphs by NOAA-PIFSC

10
Figure 5a. Percentage of mean bleaching prevalence around the Main Hawaiian Islands in 2014 and 2015
presented at the sector (coastline) scale. Data provided by the Hawai‘i Coral Bleaching Collaborative and map
made by NOAA-PIFSC.
Figure 5b. Percent coral cover lost from 2013/2015 (combined) and 2016 around the Main Hawaiian Islands
from visual estimates of % coral area. Data and map by NOAA-PIFSC.
-9.3%
-0.9%
-8.4%
-7.9%
-12.7%
-3%
-3%
1.4%
4.8%
-14.8%
-1.2%
-2.5%
0.1%
-6.5%
-1.3%
-5.4%
-2.2%
Maui
Molokai
Niihau
0 100 200
km
% Coral Area Lost
From 2013/2015 to 2016
-15 to -10
-10 to -5
-5 to 0
0 to 5
No Data
O‘ahu
Hawai‘i
Kaua‘i
Lāna‘i

11
2016
On November 3, 2016 the NOAA Coral
Reef Watch program provided an update
on the status of the temperature anomaly, which includ-
ed a La Niña Advisory [29]. Negative SST anomalies
were occurring across much of the eastern and central
equatorial Pacific Ocean, suggesting an overall cooling of
the region. It is thought that La Niña conditions will per-
sist through winter 2016-17 and it is not forecasted that
Hawai‘i will experience another coral bleaching event
during this period (as of February 2017) (Figure 6).
Figure 6. Timeline of important events during the 2014 - 2016 mass coral bleaching event in Hawai‘i
NOAA
announces
development of
El Niño event
MAR
2014
AUG
2014
OCT
2014
DEC
2014
OCT
2015
OCT
2015
NOV
2015
FEB
2016
Mass bleaching
develops in the
Northwest
Hawaiian Islands
(NWHI)
Substantial
recovery
observed in
K ˉane‘ohe Bay
Temperature
stress reaches
12˚C-Weeks in
the MHI, mass
bleaching is
observed in
West Hawai‘i
and Maui
NOAA announced
a La Niña advisory,
overall cooling
of the region, no
bleaching was
observed in
Hawai‘i
Bleaching is
observed in the
Main Hawaiian
Islands (MHI),
including
K ˉane‘ohe Bay
NOAA declares
third ever global
bleaching event
Significant loss
of coral is
documented in
West Hawai‘i

12
Section Two: Developing a
Coral Bleaching Recovery Strategy
A bleaching event can lead to a shift in the coral reef
ecosystem from a coral-dominated state to an al-
gal-dominated state. This alternative state is less desir-
able because it is generally less valuable and provides
less ecosystem services.
Coral reef decline can be permanent or temporary,
depending on its resilience, which is a reef’s ability to
absorb disturbance (e.g. a bleaching event) and respond
to change while maintaining the same function, and
thus providing the same ecosystem services
1
. Coral reef
resilience has three components: tolerance, or the ability
to survive bleaching; resistance, or the ability of corals
to withstand high temperatures without bleaching; and
recovery, or the ability of coral to be replenished after a
significant mortality event [20].
Despite the potential loss of ecosystem services, there
have been few examples worldwide of the practical
implementation of resilience principles into management
action [30, 31]. Recently, a resilience-based manage-
ment framework has been proposed, which integrates
resilience theory into coral reef management through the
identification of management ‘levers’ [32]. Levers are
management actions that will have a direct impact on
the specific management objective within the resilience
framework. However, this process identifies broad suite
of actions, or approaches, that managers could imple-
ment (e.g. ‘reduce fishing of herbivores’) and did not a)
identify specific actions that managers could take (e.g.
bag limits versus size limits, etc.) or b) prioritize these
actions on a site-specific level.
The Coral Bleaching Recovery Plan focuses on the third
aspect of coral reef resilience–recovery from a signifi-
cant mortality event. To develop a strategy to promote
coral recovery following the mass bleaching event in
Hawaii, we developed a strategy which has four steps:
1 Define the role of resource managers
2 Select priority areas for management implementation
3 Gather expert judgment on ecologically effective management actions
4 Analyze empirical evidence describing how the most highly ranked management actions
could meet our recovery objectives
Defining the role of the resource manager was needed to
understand the full array of potential actions managers
could take particularly following a mass bleaching event
as well as investigate what actions managers have pre-
viously taken. Surveying coral bleaching experts on both
a global and local scale allowed narrow the possibilities
of management action based on expert judgment, which
is a method commonly used commonly used in man-
agement decisions, especially when there is urgency to
the decision-making process or a lack of other credible
sources of information [33]. Expert judgment can be
particularly useful in situations where certain parameters
are not easily assessed (for example future conditions or
the effects of hypothetical actions) [34-37]. Despite the
usefulness of expert opinion, the use of this approach
naturally creates some uncertainty [33, 38]. The use
of scientific literature has its own level of uncertainty,
as the limitations of individual studies in terms of their
wider application, may not be thoroughly discussed [39].
For the purposes of the Coral Bleaching Recovery Plan,
we ultimately based our conclusions on management
actions that were prioritized in both expert judgment and
the literature analyses.
1
This definition refers to ‘resilience’ as described in: Holling, C. 1973. Resilience and Stability of Ecological Systems. Annual review of
Ecology and Systematics. 4.1: 1-23.
13
Part 1: Defining the Role
of Resource Managers
2
For a full description of the methods and analysis of coral bleaching literature, as well as detailed descriptions of each case study, please refer to this report:
https://dlnr.hawaii.gov/reefresponse/files/2016/09/literature-review_final-report_FINAL.pdf
The first step in the development of the Coral Bleach-
ing Recovery Plan was to review scientific literature to
define the potential role of resource managers following
a bleaching event. Primary literature and management
reports were gathered from the Coral Bleaching Working
Group, the Web of Science database, Google Scholar,
and the Reef Resilience Network. Database search
terms included ‘coral bleaching AND management’,
‘coral bleaching AND recovery’, and ‘coral bleaching
AND intervention.’
We reviewed and analyzed over 200 peer-reviewed
articles and reports categorizing management recom-
mendations and looking for intervention case studies
2
.
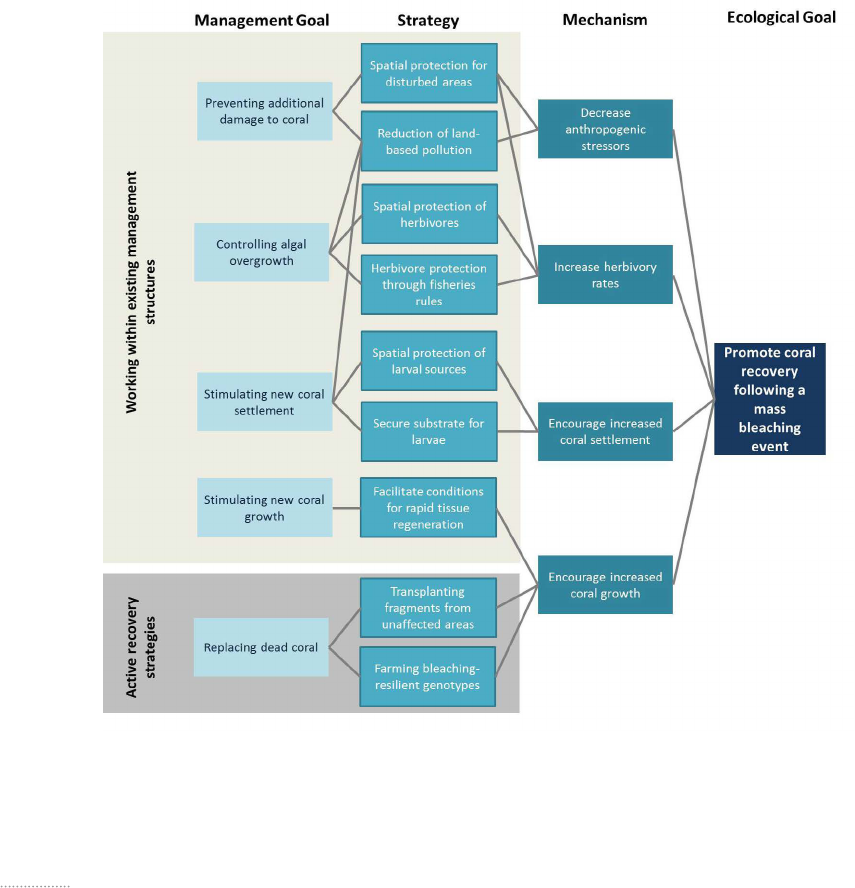
The literature analysis identified five potential goals that
resource managers could have when intervening follow-
ing a bleaching event: 1) prevent additional damage to
coral, 2) control algal overgrowth, 3) stimulate new coral
settlement, 4) stimulate coral regrowth and 5) replace
dead coral (Figure 7). The five goals each link to specific
strategies that could be used to achieve the goal, which
are additionally linked to the ecological goal through a
mechanism.
Figure 7. Illustration of the connections between the ecological and management goals following a mass bleaching event based on a re-
view of over 200 peer-reviewed scientific articles and reports. The framework depicts managers intervening in one of two broad categories:
either within existing management structures or employing active recovery strategies. Five management goals were identified that would
employ a combination of nine strategies. The strategies are linked to the ecological goal of promoting coral recovery through a mechanism.

14
‘Preventing additional damage to coral’ refers to the
management goal of protecting coral reef areas from
stressors which may compound the effect of a bleaching
event. Examples of these stressors could include over-
fishing, land-based pollution, or physical breakage from
boats, trampling, etc. ‘Controlling algal overgrowth’ refers
to the goal of preventing or potentially reversing a phase
shift from a coral-dominated system to an algal-domi-
nated system. ‘Stimulating new coral settlement’ refers
to the goal of managers creating conditions which is
conducive to coral larvae settling and eventually replac-
ing the dead coral. ‘Stimulating coral regrowth’ refers to
mangers creating conditions under which remnant coral
that has survived the bleaching event can rapidly regrow
and populate the dead area. These goals all under the
overarching category of mangers working within existing
management structures, meaning bolstering rules and
regulations that are likely ongoing and relying on natural
recovery processes. ‘Replacing dead coral’ refers to man-
agers either by growing replacement corals in a nursery
and then transplanting them to a bleaching affected area
or by transplanting healthy corals from an unaffected
area to the bleached reef. This goal falls under the active
recovery category, meaning managers would actively
intervene to physically restore bleaching affected areas.
Only a subset of the 200 reviewed papers referred to
specific management goal. Of the papers that specifically
referred to one of the management goals, the most fre-
quently recommended goals were ‘preventing additional
damage to coral’, which was recommended 42 times and
‘controlling algal overgrowth’, which was recommended
35 times. The least frequently recommended goal was
‘stimulating coral regrowth’ (Figure 8). Several papers
recommended a combination of these goals.
Figure 8.
Number of times management
goals were recommended in
the reviewed literature. Only a
subset of articles addressed a
specific management goal and
several papers recommended a
combination of these goals.
Preventing additional damage to coral allows for the natural recovery of dead or damaged
corals. In the literature, the main management action to prevent additional damage to coral
reef areas following a bleaching event was the creation of MPAs [18, 40, 41]. The need for
new management approaches for exploited areas outside of MPAs was also acknowledged
[40, 42]. It was strongly suggested that these protected areas should be placed on and
around reefs that have naturally higher resiliency to bleaching events [43-56]. Additional
actions to prevent damage included the, reduction of harmful sediment, nutrients, and
other pollutants.
Controlling algal overgrowth allows for the settlement of new coral recruits and helps to
prevent phase shifts excessive algae. Preventing overgrowth prevents reefs from becoming
dominated by algae that inhibit coral growth and recruitment (e.g. thick turfs and macroal-
gae) and increases cover of algae that are benign or inferior competitors to corals (e.g. heav-
ily cropped turfs and crustose coralline algae)–and can therefore lead to substantially better
Preventing Additional
Damage to Coral
Controlling Algal
Overgrowth

15
The literature emphasizes that not all herbivores have equal effects on rates of coral recov-
ery, and that managers should target those species, functional groups, and sizes that have
the greatest local impacts [2, 61-63]. Many researchers have focused on parrotfish (Lab-
ridae, subfamily Scarinae) and their role in the removal of algae from coral reefs following
disturbance. As with other herbivores, it has been found that their effect differs among spe-
cies, functional groups, and sizes, with larger individuals having greatest impacts on benthic
condition [2, 64]. A recent action to protect parrotfish in Belize through a fishing ban was
found to have increased the resilience of surrounding reefs six-fold [64].
Regarding specific fisheries management objectives, a recent study concluded that for
Caribbean reefs, the implementation of a harvest limit of 10% of parrotfish biomass and a
minimum size of 30cm would greatly increase coral resilience to climate change [65].
Stimulating new coral settlement is a recommended strategy for management actions en-
suring larval connectivity to the affected area. [51, 66]. It is important to ensure that larval
sources maintain a diverse gene pool to the settlement area [67]. Adequate substrate is
also imperative; measures should be taken to ensure adequate hard-bottom habitat in the
receiving site [78]. There remains a need to bring together connectivity, larval settlement,
and post-settlement mortality science to ensure that management targets the most valuable
areas [66].
Several strategies have been suggested proposed to encourage settlement of new coral to
bleached areas. For example, McLeod et al. (2009) and Magris et al. (2014) discuss the
use of MPAs to protect sources of larvae [54, 69]. Amar and Rinkevich (2007) explored the
use of active restoration to create coral nurseries as ‘larval dispersion hub.’ These farmed
colonies had 35% higher oocytes, or egg cells, per polyp and developed faster than their
natural counterparts [70]. A restoration effort in the Philippines following a dynamite blast
used plastic mesh to secure loose substrate and found that coral recruitment and percent
coral cover increased within 3 years [71]. Lastly, it has been found that early coral life stages
are particularly vulnerable to human stressors, so focusing on land-based pollution may also
be a strategy to promote settlement of coral larvae [51].
Focusing on replacing the coral killed by a bleaching event with new coral from another loca-
tion is a relatively novel active restoration method. The two main methods mentioned in the
literature are: 1) collecting fragments from unaffected areas, and 2) farming bleaching-resil-
ient genotypes to plant in the restoration area. Gomez et al. (2014) collected fragments from
unaffected reefs in the Philippines following a bleaching event and transplanted them to
the damaged area. After three years, they documented increased coral cover as well as fish
becoming attracted to the new reef [72]. This gardening method has been used extensive-
outcomes for resident corals. The majority of such studies have pointed to the protection
of herbivores, especially parrotfish, as being critical to effective management. Protection of
herbivores from fishing pressure has been projected to delay rates of coral loss even under
the most extreme bleaching and other disturbance events [57]. Where fishing pressure on
herbivores is high, two main strategies have been suggested: spatial management and the
implementation of fisheries restrictions (e.g. bag and size limits). The use of MPAs focusing
on the protection of herbivores has been cited in multiple studies as a successful strategy to
protect herbivore populations [3, 58-60].
Protecting
Herbivores
Stimulating New
Coral Settlement
Replacing
Dead Coral

16
ly in the Caribbean for the restoration of staghorn and elkhorn corals [73]. Selecting and
farming bleaching-resistant species is also a relatively new phenomenon, but it is gaining
momentum for Caribbean corals [66]. The hope is to target genotypes that are also resistant
to other stressors such as disease.
A few papers documented instances where conditions following a coral bleaching event
stimulated the rapid recovery from remnant live tissue. On the Great Barrier Reef, areas
dominated by Acropora spp. was found to recover quickly (less than one year) due to rapid
regeneration and competition with invasive algae (Lobophora variegata) [74]. Roff et al.
2014 described a phenomenon called the ‘phoenix effect,’ where small, hidden patches of
live tissue in a French Polynesia lagoon environment quickly overgrew dead coral and led to
rapid recovery of the lagoon area [75]. Finally, Graham 2013 described how if detrimental
human impacts could be reduced in the area, pulsed disturbance events could ‘jump-
start’ a return to a coral-dominated state [5]. However, all of these papers describe natural
phenomena, lacking direct management intervention. In addition, these are unique and rare
case studies and so shouldn’t be relied upon by managers as a foundational goal.
Stimulating New
Coral Growth
Of the 207 papers that were reviewed, only six examples
were found of managers directly intervening following
a bleaching event to assist in the recovery of those reef
areas (Table 1). These efforts fell into two of the man-
agement goal categories described above: 1) ‘preventing
additional damage’ to corals and 2) ‘replacing dead
coral’. It is notable that there are only a hand full of
management intervention examples and also that these
examples do not align with the majority of recommended
actions in the scientific literature (which instead point to
‘preventing additional damage’ through the implemen-
tation of MPAs and controlling algal overgrowth through
the effective management of coral reef herbivores). It
is currently unknown what prevented managers from
developing management goals that were more aligned
with the scientific recommendations. Additionally, there
was little evidence that these interventions ultimately
promoted coral recovery following the bleaching event.
Case Studies of Resource Managers Intervening Following a Bleaching Event
Table 1. Case studies of direct management interventions following a coral bleaching event
PUBLICATION LOCATION
RESOURCE
MANAGER ROLE
SPECIFIC STRATEGY
DISCUSSED
OUTCOME
TIME SCALE
OF EFFORT
Beeden et al.
2014 [76]
Great Barrier
Reef, Keppel
Islands
Great Barrier
Reef, Keppel
Islands
Malaysia,
Thailand
Preventing
additional damage
Preventing
additional damage
Preventing
additional damage
Creation of no-anchor
zones
Replacing
dead coral
Replacing
dead coral
Replacing
dead coral
Reduced anchor damage from ~80
to less than 10, coral continued to
decline
4 years
4-14
months
8 years
3.5 years
12
months
6 months
Yeemin et al.
2012 [77], Tun
et al. 2010 [78]
Closure of high-traffic
dive sites
No biological outcome could be
found, some conflict between
managers and dive site users
resulted
GBRMPA 2008
[79], Bonin et
al. 2016 [80]
Gomez et al.
2014 [72]
Philippines,
Bolinao
Tanzania
Kenya
Self-moratorium on
aquarium collecting
No biological outcome found; MPA
network supports larval dispersal
Transplantation of coral
fragments to degraded,
formerly bleached area
Transplantation of coral
fragments to degraded,
formerly bleached area
Transplantation of
bleaching-resistant
corals to formerly
bleached area
Transplantated corals were heavily
preyed upon by coral-eating fish,
which limited coral recovery
After 12 months, recorded high
survivorship (~95%), extensive
coral cover; after 16 months more
transplanted colonies were fusing
and reef fish using the new habitat
Mbije et al.
2013 [81]
After one year, saw high surviorship
of transplants, low cost showed that
transplantation could maintain
ecosystem function
McClanahan et
al. 2005 [82]

17
Part 2: Selecting
Priority Areas
Four priority areas for management intervention have
been identified, which were chosen because they had
the highest levels of either exposure to high ocean tem-
peratures and/or had the highest levels coral mortality
following the 2014/2015 bleaching event. The four
priority areas are: west Hawai‘i, leeward Maui, K ˉane‘ohe
Bay (O‘ahu) and North Kaua‘i (Figure 8). The priority
areas serve as templates for where management inter-
ventions are most needed. Hawai‘i’s coral reef scientists
and managers worked collaboratively to identify potential
management implementation obstacles and opportuni-
ties as well as research needs identified for each of the
four areas. These lists which may serve as a guide for
future management implementation (see Appendix B).
Figure 9. Priority sites for the implementation of management actions to promote coral bleaching recovery, from top left (North Kaua‘i,
K ˉane‘ohe Bay, west Maui and west Hawai‘i). These sites were chosen because they had the highest levels of exposure to high ocean
temperatures and/or the highest rates of coral mortality following the 2014/2015 coral bleaching event.
NORTH KAUA‘I
KANE‘OHE BAY
LEEWARD MAUI
WEST HAWAI‘I

18
Part 3: Gathering Expert Opinion of
Ecologically Effective Management Actions
Global Scientist Expert Judgement
In addition to understanding the potential roles that
resource managers could play and have played in pro-
moting coral recovery following a mass bleaching event,
Hawai‘i managers needed information on the perceived
1
The term ‘expert’ is based on the definition described in: Burgman, M., A. Carr, L. Godden, R. Gregory, M. McBride, L. Flander, and I. Maguire. 2011.
Redefining expertise and improving ecological judgment. Conservation Letters. 4: 81-87.
2
For a full description of the methods and analysis for the Coral Bleaching Recovery Survey, please reference this report: https://dlnr.hawaii.gov/reefre-
sponse/files/2016/09/CoralRecoverySurvey_FINAL.pdf
For the DAR online survey, global bleaching experts were defined as meeting at least one of the
following criteria:
1 Lead author on a scientific paper or article dealing with an aspect of coral bleaching or
other relevant topic (e.g. herbivory). Only the lead author was included on the contact list if
the research was conducted outside of Hawai‘i.
2 Author (lead or otherwise) of a paper/article focused on Hawai‘i dealing with an aspect of
coral bleaching or other relevant topic (e.g. herbivory).
3 Participant in a coral bleaching workshop
4 Analyze empirical evidence describing how the most highly ranked management actions
could meet our recovery objectives.
ecological effectiveness of specific actions that could
be employed to reach their recovery goals. This was
accomplished through a DAR online survey to gauge the
judgment
1
of global coral bleaching experts
2
as well as
an in-person voting exercise with Hawai‘i-based manag-
ers and scientists at an August 2016 workshop.
Based on these criteria, a list of 176 experts was devel-
oped. Those experts were asked to score the ecological
effectiveness of 22 potential management actions to
promote the recovery of bleached reefs using a weighted
point system ranging from ‘very effective’ to ‘not effec-
tive.’ The management actions were derived from a
review of the literature described in Part 2, suggestions
from local experts, previously identified actions from
a 2013 Hawai‘i coral bleaching response workshop of
resource managers and scientists, restoration strategies
that Hawai‘i DAR already engage in, and actions that
had been suggested by stakeholders following the 2015
bleaching event (Table 2). These actions fit into the
framework that was developed in Part 2, as practical
ways that mechanisms will lead to the ecological goal of
promoting coral recovery (Figure 10).

19
Table 2. Management actions that were selected to be in the coral bleaching recovery survey. These actions were derived from a review
of the literature described in Part 2, suggestions from local experts, previously identified actions from a 2013 Hawai‘i coral bleaching
response workshop of resource managers and scientists, restoration strategies that Hawai‘i DAR already engage in, and actions that had
been suggested by stakeholders following the 2015 bleaching event.

Figure 10. Revised management framework with the inclusion of potential practical actions
that could be taken to promote recovery following a mass bleaching event.
21
The online global survey received 82 complete
responses (47% response rate). Respondents were
based in 12 countries; the majority being either
American or Australian. The majority (52%) had more
than 10 publications in the field and 72% had more
than 10 years of experience.
We ranked the management actions using their weight-
ed group average score. This simple method provides
accurate judgments compared with more complex
methods [83]. The management action with the highest
average effectiveness score from the survey was ‘reduce
sediment stress on coral reefs by implementing addition-
al land-based mitigation in adjacent watersheds’ (Figure
11). The most common comments added by survey
takers related to reducing sediment was to emphasize
that this was a critical action, but also that it was very
complicated to achieve and may only be effective in
certain systems. Other of the top five actions were:
‘reducing nutrients’, ‘enhancing enforcement’, ‘creating
permanent no-take areas through a network of MPAs’,
and ‘creating a network of herbivore protection areas’.
Related to MPAs, respondents added comments reflect-
ing that this was only part of the necessary response,
and that effective management of these areas would
be key to their success. Comments related to spatial
management of herbivore populations indicated that
managers should look at the success of local herbivore
protection areas first and that herbivore management
should be prioritized in areas where the threat of algae
growth is greatest. The management strategies with
the lowest scores were: ‘create artificial reefs in heavily
bleaching-impacted reef areas’, ‘attempt to eradicate in-
troduced fish species such as Roi’, ‘establish a network
of temporary, rotationally closed, no-take MPAs’, and ‘es-
tablish a temporary moratorium on aquarium collecting.’

22
Figure 11. Management strategies from the global expert survey ranked by ecological effectiveness,
showing total of weighted responses.
23
Hawai‘i-based Scientist and
Manager Expert Opinion
Hawai‘i-based scientist and manager expert opinion
was gathered through a workshop in August 2016 in
Honolulu. The 44 participants included Hawai‘i-based
representatives from DAR, NOAA, the Hawai‘i Institute
of Marine Biology (HIMB), the University of Hawai‘i
(UH), The Nature Conservancy (TNC), and Conservation
International (CI).
To develop a list of ecologically effective statewide man-
agement recommendations, the workshop group was
provided with the 22 potential management actions from
the global survey. Participants were also provided with
the results of the global Coral Bleaching Recovery Sur-
vey, and summary information on perceived ecological
effectiveness of each action. Each participant was then
given five points to vote for the most effective actions.
Participants could use all five points for one action, or
distribute their votes among several actions, but could
only use up to five votes.
The management action that received the most points
(i.e. “most effective”) was ‘establish a network of per-
manent, fully protected no-take Marine Protected Areas
(MPAs)’, which received 50 points (this action ranked 4
in the global survey). Another top-ranked action was to
‘reduce land-based pollution’, which the group dis-
cussed as encompassing both sediment and nutrient
stress on coral reefs. ‘Herbivore management’ was the
third highest prioritized action, which the group decid-
ed should encompass a combination of management
actions. Lowest ranked actions again included ‘create
artificial reefs in heavily bleaching- impacted reef areas’
and ‘attempt to eradicate introduced fish species such
as the Roi, or Peacock Grouper, Cephalopholis argus.’
Related to herbivore management, the participants
could not hone in on one specific management action
or list of important species, but deemed it crucial to
conduct research to examine the relative influence of
herbivores in the affected areas including both fish and
invertebrates. The group also felt that adding the devel-
opment of a strategic communication plan to commu-
nicate resilience science and promote individual action
should be added. These results concurred with the
results from the global survey asking for expert opinion
on these same management actions. Although in slightly
different order, the reduction of land-based stressors,
establishment of MPAs, and focus on herbivore manage-
ment were consistently cited as ecologically effective ac-
tions that managers could take to promote coral recovery
and resilience following a bleaching event.
This exercise allowed us to compare the Hawai‘i-based
expert judgment to the online survey of global expert
judgment. Although the two assessments had different
methods (a weighted average score versus total number
of points), we can compare across methods by looking
at how each management action ranked in terms of
their overall effectiveness. We did this by giving each
rank position a point score. Actions that were in a more
highly ranked position received more points. Points were
then summed to provide a “combined ranking” based
on both the Honolulu workshop and the global survey.
This slightly altered the top actions, ultimately providing
a succinct list of the top-ranked management actions
based on global and Hawai‘i-based expert judgment.
(Table 3). There were two instances of ties in this pro-
cess. Actions with tied numbers of points shared that
ranking position. Based on this ranking, we honed in on
the top ten rated actions (ranked positions 1-9 with a tie
for second position).

24
Table 3. Ranking of management actions based on expert judgment from a global survey and Hawai‘i workshop, indi-
cating the top 10 ranked actions. Actions were compared by giving each rank position a point score, meaning actions
that were in a more highly ranked position received more points. Points were then summed to provide a “combined
ranking” based on both the Honolulu workshop and the global survey.

25
Part 4: Critically Analyzing the Effectiveness
of Top-Ranked Management Actions
To strengthen our identification of effective management
actions, we further investigated the top ten actions from
the expert judgment rankings using scientific literature.
This allowed us to minimize the potential biases that
could come from each type of analysis and provided us
with a final ranking based on both types of analyses.
Following the Honolulu workshop, we added two man-
agement actions (‘prohibit use of laynets statewide’ and
‘prohibit use of SCUBA spearfishing’) because they were
continuously raised in workshop discussion and subse-
quent meetings. However, because these actions were
not included in the expert judgment rankings, they were
included in the analysis of scientific literature, but not in
the final ranking.
To rank the potential management actions, we first
collected primary literature and reports using the search
format “[management action] AND coral recovery” for
each of the twelve actions from Google Scholar as well
as the Web of Science databases. Papers were includ-
ed in the analysis if they were specifically relevant in
answering whether each action is effective in terms of
a) the action’s management objective and b) our overall
coral bleaching recovery objective. For example, related
to ‘reduce sediment stress on coral reefs by imple-
menting additional land-based mitigation in adjacent
watersheds’, papers were included that described the
ability of watershed mitigation to reduce sediment (the
management objective) as well as the ability of corals to
recover once a reduction in sediment as occurred (the
recovery objective).
Once the papers were collected the evidence was cate-
gorized into one of six types, which describe whether the
evidence was empirical (based on direct observation) or
theoretical (based on theories or models), whether the
research from inside or outside of Hawai‘i, and if it had
been assessed at a global scale at multiple sites. The
categories were then weighted, which valued empirical
evidence over theoretical, research from Hawai‘i over
research from outside Hawai‘i, and highly valued global
studies with multiple sites (Table 4).
Table 4.
Point values for categories of
evidence describing the ability
and limitations of manage-
ment actions to achieve their
management and recovery ob-
jectives. This scale was used to
categorize and score scientific
literature.

26
Over 100 additional papers were reviewed for this
portion of the analysis. Evidence varied by point cate-
gory and also was variable throughout the management
actions (Appendix C). Each piece of evidence from these
papers was categorized into one of the evidence cate-
gories for both the management and recovery objective.
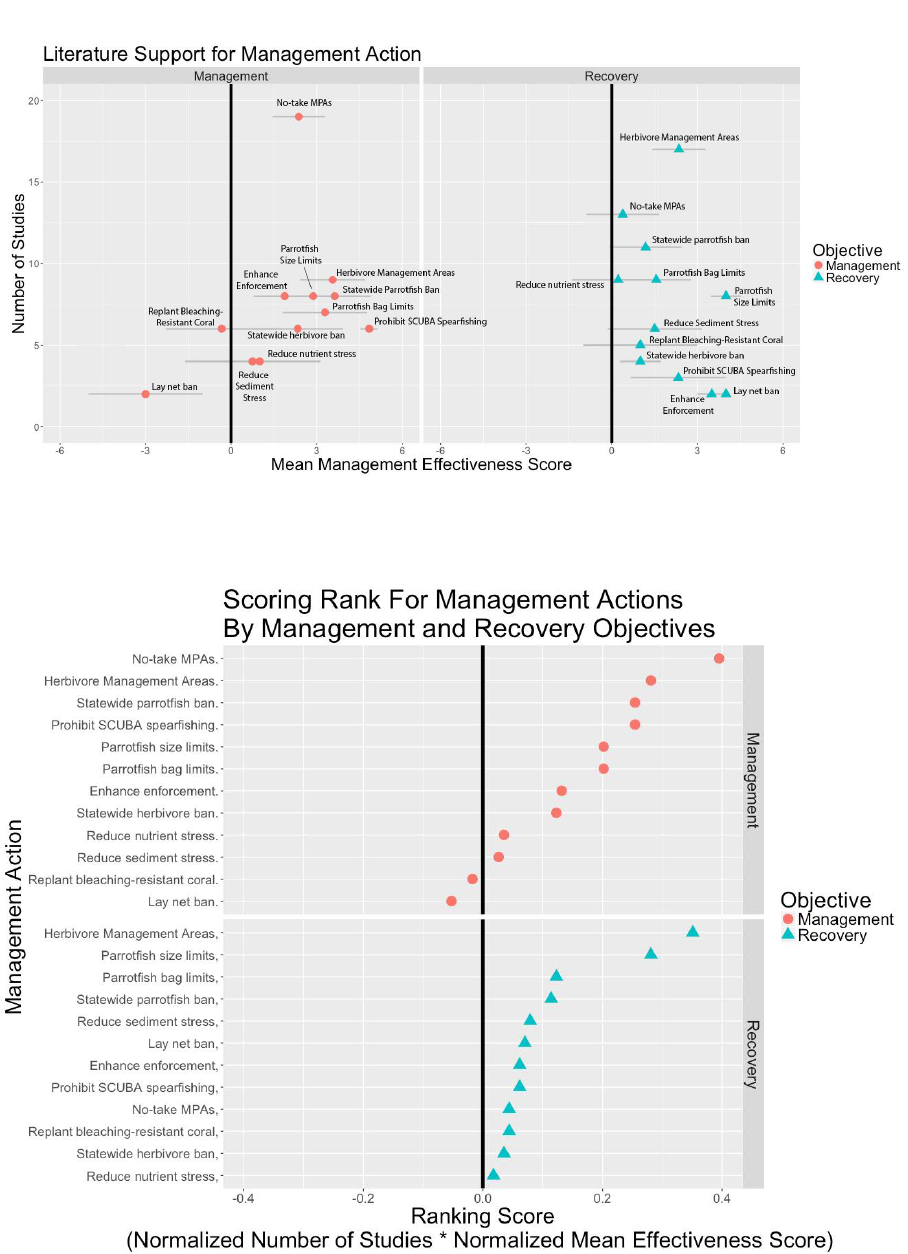
To rank the effectiveness of each management action
based scientific evidence, we first calculated the average
score of each action’s management objective and recov-
ery objective. We then plotted the average score against
the number of studies that this average represents,
or the literature support for each management action
(Appendix D1). We calculated the management and
recovery scores for each action by normalizing the num-
ber of studies and the mean effectiveness score, then
multiplying these metrics (Appendix D2). This allowed us
to consider each action’s effectiveness and our certainty
in this effectiveness, based on the number of studies.
Lastly, we summed the management and recovery
ranking score to give us our final, combined score for
each management action. This produced a quantitative
ranking of the management actions considering their
management and recovery effectiveness and the certain-
ty of this effectiveness (Figure 12).
Figure 12. Top-ranked management actions from the expert judgment surveys re-ranked by the sum of manage-
ment and recovery objective ranking scores. The summed scores take into account the ability of each man-
agement action to meet its management and coral bleaching recovery objective as well as the certainty of that
effectiveness, based on the number of studies.
Using this method, we found that ‘establishing perma-
nent HFMAs’ had the highest summed ranking score,
followed by ‘establish size limits to protect parrotfishes’,
and ‘establish a network of permanent, no-take MPAs.’
The lowest ranking actions were ‘prohibit all use of lay
nets’, ‘identify, collect, propagate and replant corals
found to be resistant to bleaching’, and ‘reduce
nutrient stress.’ The next section summarizes the evi-
dence for each management action that was included in
this analysis.
A full description of each action’s management and
recovery objective as well as the categorization of all
limiting and supporting evidence can be found in
Appendix E.

27
Spatial Management
Fisheries Rules
Globally and in Hawai‘i, no-take MPAs have been found to have both fisheries and ecosys-
tem benefits [84-92]. MPAs have been critical in maintaining coral cover over time (but not
necessarily increasing it) and in some have prevented cases, preventing algal overgrowth
[93-99]. However, MPAs in Hawai‘i have limitations especially when they are too small and
don’t represent a diversity of habitats ([96]. When MPAs were evaluated against various
potential management goals, there is a weak connection specifically between no-take MPAs
and coral recovery [92]. Regional environmental and habitat variability also strongly affect
the success of an MPA in a given location [100-103] and therefore strategic placement of
MPAs is crucial.
Lay nets have been proven to be destructive to benthic environment when they become
entangled in coral and cause physical damage [108, 109]. There has only been one study
which explored the relationship of lay nets to recovery from coral bleaching events (via their
effect on herbivore populations) and found that lay nets were not in the top gear types for
herbivore catch [110]. A study from Moloka‘i, Hawai‘i concurred with these findings and
found that herbivores only constituted a minimal percentage of the total number of fish
caught and therefore banning their use would likely not have a great effect on herbivore
populations [111]. It is important to note here that there has been relatively very few studies
connecting lay net fishing to herbivores or to coral recovery. It is also possible that the
Moloka‘i study captured local-scale patterns and may or may not represent the larger area.
Parrotfish management in Hawai‘i could be greatly enhanced by banning spearfishing with
SCUBA, especially at night, as herbivores including parrotfishes and surgeonfishes are
primary components of the spearfishing catch in Hawai‘i and coral reef fishes, particularly
parrotfishes, are more vulnerable at night [96, 116, 117]. Surveyed fishermen in Hawai‘i felt
that SCUBA diving allows for inappropriate levels of fishing efficiency [114]. As with laynets,
there has only been one study that explored the relationship of fishing gear to recovery
from coral bleaching events. In general it is thought that gear restrictions that protect large,
grazing species would assist in maximizing algal removal [110]. In addition, spearfishers in
Kenya were found to cause the highest rates of physical damage to coral when fishing [108].
HFMAs have been successful in increasing herbivore biomass within their boundaries in
Hawai‘i. In the first six years of the Kahekili HFMA (KHFMA), mean parrotfish and surgeon-
fish biomass both increased within the KHFMA by 139% and 28% respectively, however
this was mostly seen in small to medium sized species, whereas large-bodied species have
not recovered, likely due to low levels of poaching of preferred fishery targets [60]. Addi-
tionally, macroalgal cover has remained low and coral cover stabilized with a slight increase
from 2012 through early 2015 (before the bleaching event) [60]. The Redlip Parrotfish
(Scarus rubroviolaceus), a critically important parrotfish in Hawai‘i has qualities that make
them a good candidate for management through MPAs [104]. However, like no-take MPAs
there will be variability in its success based on the capacity of individual reef areas to sup-
port herbivores [100]. Spatial management has been found to have a strong connection to
the mechanism of herbivory and its role in shaping benthic communities, however this role
has not been completely shown to lead coral recovery [92]. Like no-take MPAs, regional
variability will strongly affect their success [105-107].
No-take MPAs
Prohibit use of
lay nets
Prohibit all use
of SCUBA for
spearfishing
Establish HFMAs

28
There are numerous studies demonstrating the sensitivity of herbivore populations to over-
fishing [115 - 117]. There is evidence of overfishing of herbivores in Hawai‘i [96, 116-118].
A parrotfish fishing ban in Belize has reduced herbivorous fish harvest and had a high com-
pliance [119, 120]. Related to coral recovery, fished reefs with fewer herbivores have a great-
er chance of being overgrown by algae [5]. In addition, the parrotfish ban in Belize resulted
in increased coral resilience [64]. However, as with spatial management, it is unlikely that
all reef areas will respond similarly to an herbivorous fish ban [105-107, 117]. For example,
an assessment in New Caledonia concluded that a ban on herbivore harvesting would be
unlikely to improve coral reef resilience based on local conditions [121].
For parrotfish specifically and especially male fish, there is evidence from Belize that
populations can recover quickly from overfishing following a complete ban [122]. Parrotfish
play multiple ecological functions in coral recovery, including controlling algal overgrowth
and creating new space for coral settlement [123, 124] and these relationships have been
identified in Hawai‘i [125]. Specifically, scrapers (Chlorurus spilurus, Bullethead Parrotfish;
Chlorurus perspicillatus, Spectacled Parrotfish; and Scarus rubroviolaceus, Ember par-
rotfish) were most strongly associated with Hawai‘i reefs being in a coral-dominated state
[126]. However, like the complete ban on herbivorous fishing and spatial management,
success will vary depending on geographical factors [105-107, 117].
Very specific minimum size limits have been identified for Hawai‘i in order to protect pop-
ulations from overfishing [127]. Specifically, DeMartini et al. 2016 suggested the minimum
legal sizes of parrotfishes in Hawai‘i should increase to 35.6 cm (14 inches) LF for the two
large-bodied species (Scarus rubroviolaceus and Chlorurus perspicillatus) and 24.3 cm (11
inches) LF for Calotomus carolinus. Because the bioerosion abilities of parrotfish increase
with size, protecting larger parrotfish will compound their ability to aid in coral recovery
processes [65,125, 128]. Because there are natural differences in the capacity of specific
reef areas to support herbivores, size limits may not have a consistent effect across all sites
[105-107, 117].
Bag limits would essentially equate to a partial ban on parrotfish harvest, and therefore have
many of the same benefits, but likely with less impact. In Hawai‘i, it has been suggested that
prohibiting the take of blue/green male parrotfishes would be effective at protecting against
overfishing of sex-changed male fish [128]. As with total protection, the natural differences
in the capacity of different reef areas to support herbivores, will mean that bag limits will not
have a consistent effect across all sites [106-108, 118].
In general, it is clear that excessive sediment has negative effects on coral, and prevents
reefs from returning to pre-impact conditions [129]. Reducing sediment through watershed
management has been successful in many island nations and at a large scale in China
[130, 131]. However, a global review found only one example of reductions in net fluxes of
land-based sediment levels following restoration efforts [132]. There is an established rela-
tionship between the health of watersheds and the health of adjacent reefs in Hawai‘i [133],
however if sources of sediment are chronic it is unlikely that corals will be able to rapidly
recover after restoration actions [132].
Prohibit all take
(commercial and
noncommercial) of
herbivorous fish
Prohibit all take
(commercial and
non-commercial)
of parrotfish
Establish size
limits to protect
parrotfishes
Establish bag
limits to protect
parrotfishes
Partner with other
agencies to reduce
sediment stress
through land-based
watershed mitigation
Land-based Strategies

29
In general, elevated nutrient levels have a negative effect on corals [129]. Diverting nutrients
has led to coral reef recovery in Hawai‘i (K ˉane‘ohe Bay) [134], though this is only known ex-
ample of this type of ecosystem reversal [132]. As with reducing sediment, watershed man-
agement has been successful in many island nations [130]. However, also as with reducing
sediment, it is unlikely that corals will quickly respond to reductions in nutrients where there
remains chronic exposure to other forms of land-based pollution [42, 132].
Partner with other
agencies to reduce
nutrient stress
through land-based
watershed mitigation
Several coral transplantation efforts have recorded high survivorship of transplanted corals
at a relatively low cost to managers, indicating that such an approach may enhance reef
recovery [72, 81, 135–137]. A pilot project on the Great Barrier Reef moved corals associ-
ated with relatively warm conditions to cooler conditions. This effort proved successful when
evidence of recruitment was found, but it was only found at certain locations [138]. There
have also been examples of efforts that were not successful in transplanting bleaching-resis-
tant corals, often suffering from logistical challenges [66, 82]. Additionally, one study found
corals lost their bleaching resistant ‘edge’ once they were planted in a new location [139].
Finally, there are some ethical concerns about moving corals including the potential for
‘outbreeding depression’ and the spread of disease into the receiving area [66,140].
Adequate enforcement is often correlated with high fish biomass and richness on a global
scale [141, 142]. In Hawai‘i’s Community Fishery Enforcement Unit (CFEU)’s first year of
operations (2013-2014), officers issued a number of citations including net, diving, lobster,
undersized fish, and bag limit violations [143]. Enforcement has been cited as a critical
component of MPA management specifically [98, 142, 144, 145] and can prove cost-ef-
fective when compared to active restoration [146]. Limiting factors include that levels of
enforcement are rarely quantified or reported [98] and the fact that there are a number of
specific and distinct actions that could be taken to increase compliance [147], and so a
locally-appropriate strategy must be developed.
Identify, collect,
propagate and
replant corals found
to be resistant to
bleaching
Enhance marine
enforcement efforts
to ensure the
effectiveness of rules
relating to coral reef
protection
Aquaculture Techniques
Other

30
Section Three: Conclusions
The goal of the Coral Bleaching Recovery Plan is to pro-
mote coral reef recovery following the 2014-2015 global
coral bleaching event. The bleaching event had effects
throughout the state of Hawai‘i and its severity warranted
management intervention. This is especially true in the
four priority sites, of north Kaua‘i, K ˉane‘ohe Bay, leeward
Maui, and west Hawai‘i which had the highest level of
either exposure to high ocean temperatures or coral
mortality following the bleaching event. This plan will aid
managers in implementing effective management ac-
tions by prioritizing which potential management actions
would be the most ecologically effective in promoting re-
covery. This was answered by collecting expert judgment
from both global and local scientists and managers as
well as by critically analyzing the scientific literature on
the applications of those actions.
Comparing results from
expert judgment and the
literature analysis
We did this by giving each rank position a point value
and then summing these values for the three analysis
types. Prohibiting SCUBA spearfishing and prohibiting
laynets were removed from this comparison because
they were not present in the expert judgment assess-
Although the global online survey, the workshop exer-
cise, and the literature analysis were conducted using
different methods, we can compare their results by
looking at how management actions were ranked relative
to each other across the three activities (Table 5).
Table 5. Comparison in the relative ranking of management actions between the global online survey,
the Hawai‘i workshop voting exercise, and the literature analysis

31
ments. It is clear from this comparison that there was a
substantial difference in the rankings between the expert
judgment and the literature analysis for a portion of the
management actions. For example, ‘reducing sediment
stress’ ranked first and second from expert judgments
methods. However, it ranked ninth (third to last) in the lit-
erature analysis. This may be because experts were asked
to not base judgments on the feasibility of a given action.
Thus, it is reasonable to conclude that, if it were possible
to decrease sediment, that this would be effective-
because of the known negative link between sediment
and coral survival. However, the literature analysis
identified only one example of successful watershed
management leading to reduced sediment fluxes on a
Limitations of this analysis
Management implications
and next steps
The limitations of basing policy decisions on expert judg-
ment or scientific literature alone have been discussed in
a previous section. To overcome these potential biases, we
combined the rankings from both types of analyses and
based on conclusions on their collective findings.
Additionally, our evaluation did not consider parameters
which are likely to affect the effectiveness of a particular
action, for example management feasibility, enforceability,
implementation cost, man hours required, sociocultural
cost, or public opinion. We assume here that all man-
agement actions are equally feasible and enforceable.
However, in developing a management strategy, it is criti-
cal that these factors be considered. However, beginning
with an evaluation based on ecological effectiveness will
ultimately strengthen the overall assessment of the
potential management actions. Finally, our analysis
focused on the ability of management actions to promote
coral recovery following a bleaching event. Although
there may be some inherent overlap, this analysis did not
evaluate how the actions could impact the resistance of
Hawai‘i’s coral reefs to future climatic disturbances. This
will be a critical piece of the ultimate recovery strategy.
Establishing a network of permanent no-take MPAs and
establishing a network of Herbivore Fishery Management
Areas (HFMA) were highly ranked actions, which per-
formed well in both the expert judgment assessments and
the literature analysis. Our analysis therefore indicates
that spatial management, particularly herbivore man-
agement, is critical to coral recovery in Hawai‘i. Spatial
management should also target areas with a high natural
resiliency and recovery potential. Enhancing enforcement
also scored well across all analyses but additional investi-
gation is needed to inform what type of action (increasing
education, increasing penalties, increasing number of
officers) would be the most impactful. Lastly, fisheries
rules, especially pertaining to parrotfish are particularly
important component of any recovery action in Hawai‘i, as
shown by the detailed information on the contributions of
individual species and size classes, and the performance
of complete and partial bans in other regions of the world.
The next step in the coral bleaching recovery planning
process should be to evaluate where the top-ranked
actions including spatial management and perhaps a se-
lection of fisheries rules would have the greatest positive
impact in terms of coral reef recovery. This is still an open
question because, as the literature emphasized, man-
agement actions will not have a consistent effect based
on the natural ecological variability among different reef
areas. This evaluation should consider minimizing social
cost and consider the management feasibility of actions
that are seriously being considered for implementation.
This exercise should also be extended to consider which
management actions are the most effective in enhancing
the resiliency of Hawai‘i’s reefs to future climatic events.
large scale and ultimately coral recovery. This partly
resulted in it receiving a very low rank when using the
literature analysis method.
A second example is establishing bag limits for
parrotfishes. This ranked 9th (close to the middle of
all 22 management action options) in both the global
online survey and the Hawai‘i workshop but was the top
action in the literature analysis. What helped it rise in the
literature analysis was the fact that we have very specific
information on Hawai‘i parrotfish from DeMartini et al.
2016 which describes the positive effect that a partial
ban would have on specific species. This can be said
for the majority of fisheries rules including parrotfish size
limits and the parrotfish ban.

32
Appendix
Appendix A NOAA Coral Reef Watch Program a) plots of Sea Surface Temperature (SST), Degree Heating Weeks
(DHW), and coral bleaching alerts for 2014 and 2015 in more northerly Main Hawaiian Islands and b) maps of DHW
in the Main Hawaiian Islands region.
a)

33
b)
Appendix B a) Management obstacles (in red) and opportunities (in green) identified for implementing management
actions in the priority sites. b) Research needs in each priority site for effective management action implementation.
a)
34
b)
Appendix C The distribution of evidence in the literature analysis in each point value category and for each
management action.
35
Appendix D.1 The literature support for the top-ranked management actions based on their mean effectiveness score
and the number of studies.
Appendix D.2 The scoring rank for the top-ranked management actions based on their ranking score.

Appendix E Summary of evidence related to the ability of each action to meet its management objectives (in dark blue) and the recovery objective of promoting its
recovery objective (in light blue).
36

37

38

Coral recovery
due to
decrease in
sedimentation
becasue of
watershed
mitigation
39

40
1. Bellwood, D., T. Hughes, C. Folke, and M. Nyström. 2004. Confronting the Coral Reef Crisis. Nature. 429.6994:
827–833.
2. Bellwood, D., T. Hughes, and A. Hoey. 2006. Sleeping Functional Group Drives Coral-Reef Recovery. Current
Biology. 16.24: 2434–39.
3. Hughes, T., M. Rodrigues, D. Bellwood, D. Ceccarelli, O. Hoegh-Guldberg, L. McCook, N. Moltschaniwskyj, M. S.
Pratchett, R. S. Steneck, and B. Willis. 2007. Phase Shifts, Herbivory, and the Resilience of Coral Reefs to Climate
Change. Current Biology. 17.4: 360–65.
4. Hughes, T., N. Graham, J. Jackson, P. Mumby, and R. Steneck. 2010. Rising to the Challenge of Sustaining Coral Reef
Resilience. Trends in Ecology & Evolution. 25.11: 633–42. doi:10.1016/j.tree.2010.07.011.
5. Graham, N., D. Bellwood, J. Cinner, T. Hughes, A. Norström, and M. Nyström. 2013. “Managing Resilience to Reverse
Phase Shifts in Coral Reefs.” Frontiers in Ecology and the Environment . 11.10: 541–48. doi:10.1890/120305.
6. Ainsworth, C. and P. Mumby. 2015. Coral-Algal Phase Shifts Alter Fish Communities and Reduce Fisheries
Production. Global Change Biology. 21.1
7. Hoegh-Guldberg, O. 1999. Climate Change, Coral Bleaching and the Future of the World’s Coral Reefs. Marine and
Freshwater Research. 50. 8: 839.
8. Baker, A., P. Glynn, and B. Riegl. 2008. Climate Change and Coral Reef Bleaching: An Ecological Assessment of Long
Term Impacts, Recovery Trends and Future Outlook. Estuarine, Coastal and Shelf Science. 80. 4: 435–71.
9. Ateweberhan, M., D. Feary, S. Keshavmurthy, A. Chen, M. Schleyer, and C. Sheppard. 2013. Climate Change Impacts
on Coral Reefs: Synergies with Local Effects, Possibilities for Acclimation, and Management Implications. Marine Poll
tion Bulletin. 74: 526–39.
10. Frieler, K., M. Meinshausen, A. Golly, M. Mengel, K. Lebek, S. Donner, and O. Hoegh-Guldberg. 2012. Limiting Global
Warming to 2 °C Is Unlikely to Save Most Coral Reefs. Nature Climate Change 3.2: 165–70.
11. Ateweberhan, M., D. Feary, S. Keshavmurthy, A. Chen, M. Schleyer, and C. Sheppard. 2013. Climate Change Impacts
on Coral Reefs: Synergies with Local Effects, Possibilities for Acclimation, and Management Implications. Marine Pollu-
tion Bulletin 74.2: 526–39. doi:10.1016/j.marpolbul.2013.06.011.
12. Coles, S., and P. Jokiel. 1978. Synergistic Effects of Temperature, Salinity and Light on the Hermatypic Coral Monitpora
Verrucossa. Marine Biology. 49: 187–95.
13. Putnam, H., and R. Gates. 2015. Preconditioning in the Reef-Building Coral Pocillopora Damicornis and the Potential
for Trans-Generational Acclimatization in Coral Larvae under Future Climate Change Conditions. Journal of
Experimental Biology. 218.15: 2365–2372.
14. Gates, R., G. Baghdasarian, and L. Muscatine. 1992. Temperature Stress Causes Host Cell Detachment in Symbiotic
Cnidarians: Implications for Coral Bleaching. The Biological Bulletin. 182. 3: 324–332.
15. Grottoli, A., L. Rodrigues, and J. Palardy. 2006. Heterotrophic Plasticity and Resilience in Bleached Corals. Nature.
440.7088: 1186–89.
16. Jokiel, P., and E. Brown. 2004. Global Warming, Regional Trends and Inshore Environmental Conditions Influence
Coral Bleaching in Hawaii. Global Change Biology. 10.10: 1627–41.
17. McClanahan, T., S. Donner, J. Maynard, M. MacNeil, N. Graham, J. Maina, A. Baker, J. Alemu, M. Beger,
S. Campbell, E. Darling, C. Eakin, S. Heron, S. Jupiter, C. Lundquist, E. McLeod, P. Mumby, M. Paddack, E. Selig,
R. Woesik. 2012. Prioritizing Key Resilience Indicators to Support Coral Reef Management in a Changing Climate.
Edited by Richard K. F. Unsworth. PLoS ONE. 7.8: e42884. doi:10.1371/journal.pone.0042884.
18. Aeby, G., M. Hutchinson, and P. MacGowan. 2009. Hawaii’s Rapid Response Contingency Plan for Events of Coral
Bleaching, Disease or Crown-of-Thorns Starfish Outbreaks. The Division of Aquatic Resources, Department of Land
and Natural Resources.
Literature Cited
41
19. Baker, A., P. Glynn, and B. Riegl. 2008. Climate Change and Coral Reef Bleaching: An Ecological Assessment of
Long-Term Impacts, Recovery Trends and Future Outlook. Estuarine, Coastal and Shelf Science. 80.4: 435–71.
20. Marshall, P., and H. Schuttenberg. 2004. A Reef Manager’s Guide to Coral Bleaching. Townsville, Qld.: Great Barrier
Reef Marine Park Authority.
21. NOAA, Coral Reef Watch. 2014. Pacific Climate Update: Coral Bleaching Thermal Stress Analysis and Seasonal
Guidance through February 2015. https://coralreefwatch.noaa.gov/satellite/analyses_guidance/pacific_climate_up-
dates/pacific_climate_update_coral_reef_watch_20141103.pdf.
22. Couch, C., J. Burns, K. Steward, T. Gutlay, G. Liu, E. Geiger, C. Eakin, R.K. 2016. Causes and consequences of the
2014 mass coral bleaching event in Papahaˉ naumokuaˉkea Marine National Monument. Technical Report for NOAA
Papahaˉ naumokuaˉ kea Marine National Monument. 28pp.
23. DAR (Division of Aquatic Resources). 2014. Coral Bleaching 2014: Important Findings.
http://dlnr.hawaii.gov/reefresponse/current-rapid-responses/coral-bleaching-2014/.
24. Bahr, K., P. Jokiel, and K. Rodgers. 2015. The 2014 Coral Bleaching and Freshwater Flood Events in Kaˉ ne‘ohe Bay,
Hawai‘i. PeerJ. 3: e1136. doi:10.7717/peerj.1136.
25. NOAA, Coral Reef Watch. 2015. NOAA Declares Third Ever Global Coral Bleaching Event.
http://www.noaanews.noaa.gov/stories2015/100815-noaa-declares-third-ever-global-coral-bleaching-event.html.
26. Kramer, K., S. Cotton , M. Lamson , W. Walsh. 2016. Bleaching and catastrophic mortality of reef-building corals along
west Hawai‘i island: findings and future directions. Proceedings of the 13th International Coral Reef Symposium,
Honolulu: 229-241.
27. TNC (The Nature Conservancy). 2015. Summary of Findings: 2015 Coral Bleaching Surveys: South Kohala,
North Kona.
28. Sparks, R. 2016. Department of Land and Natural Resources, Division of Aquatic Resources. Unpublished data.
29. NOAA, Coral Reef Watch. 2016. Pacific Climate Update: Coral Bleaching Thermal Stress Analysis and Seasonal
Guidance through February 2017. https://coralreefwatch.noaa.gov/satellite/analyses_guidance/pacific_climate_up-
dates/pacific_climate_update_coral_reef_watch_20161103.pdf
30. Heller, N., and E. Zavaleta. 2009. Biodiversity Management in the Face of Climate Change: A Review of 22 Years of
Recommendations. Biological Conservation 142, no. 1: 14–32.
31. Maynard, J., P. Marshall, J. Johnson, and S. Harman. 2010. Building Resilience into Practical Conservation:
Identifying Local Management Responses to Global Climate Change in the Southern Great Barrier Reef. Coral Reefs
29.2: 381–91. doi:10.1007/s00338-010-0603-8.
32. Anthony, K., P. Marshall, A. Abdulla, R. Beeden, C. Bergh, R. Black, M. Eakin, E. Game, N. Graham, A. Green,
S. Heron, R. Hooidonk, C. Knowland, S. Mangubhai, N. Marshall, J. Maynard, P. McGinnity, E. McLeod, P. Mumby,
M. Nystrom, D. Obura, J. Oliver, H. Possingham, R. Pressey, G. Rowlands, J. Tamelander, D. Wachenfeld, S. Wear.
2015. Operationalizing Resilience for Adaptive Coral Reef Management under Global Environmental Change. Global
Change Biology. 21.1: 48–61.
33. Martin, T., M. Burgman, F. Fidler, P. Kuhnert, S. Low-Choy, M. Mcbride, and K. Mengersen. 2012. Eliciting Expert
Knowledge in Conservation Science: Elicitation of Expert Knowledge. Conservation Biology. 26.1:29–38.
doi:10.1111/j.1523-1739.2011.01806.
34. Crome, F., M. Thomas, and L. Moore. 1996. A novel Bayesian approach to assessing impacts of rain forest logging.
Ecological Applications. 6:1104–1123.
35. Marcot, B. 2006. Characterizing species at risk I: modeling rare species under the northwest forest plan. Ecology and
Society. 11: http://www.ecologyandsociety. org/vol11/iss12/art12/. (accessed November 2011).
36. Griffiths, S., P. Kuhnert, W. Venables, and S. Blaber. 2007. Estimating abundance of pelagic fishes using gillnet catch
data in data-limited fisheries: a Bayesian approach. Canadian Journal of Fisheries and Aquatic Sciences.
64:1019–1033.
37. 37. Rothlisberger, J., D. Lodge, R. Cooke, and D. Finnoff. 2010. Future declines of the binational Laurentian Great
Lakes fisheries: the importance of environmental and cultural change. Frontiers in Ecology and the Environment.
8:239–244.
42
38. Morgan, M. Granger. 2014. Use (and Abuse) of Expert Elicitation in Support of Decision Making for Public Policy.
Proceedings of the National Academy of Sciences. 111.20: 7176–7184.
39. Ioannidis, J. 2007. Limitations Are Not Properly Acknowledged in the Scientific Literature. Journal of Clinical
Epidemiology. 60.4: 324–29. doi:10.1016/j.jclinepi.2006.09.011.
40. Dodge, R., and C. Birkeland. 2008. A Call to Action for Coral Reefs. Science. 322.5899: 189.
41. Rogers, A., A. Harborne, C. Brown, Y. Bozec, C. Castro, I. Chollett, K. Hock, C. Knowland, A. Marshell, J. Ortiz,
T. Razak, G. Roff, J. Samper-Villarreal, M. Saunders, N. Wolff, P. Mumby. 2015. Anticipative Management for Coral
Reef Ecosystem Services in the 21st Century. Global Change Biology 21.2: 504–14.
42. Mumby, P., and R. Steneck. 2008. Coral Reef Management and Conservation in Light of Rapidly Evolving Ecological
Paradigms. Trends in Ecology & Evolution. 23.10: 555–63.
43. Wilkinson, C. 1992. Coral Reefs of the World Are Facing Widespread Devastation: Can We Prevent This through
Sustainable Management Practices? Proceedings of the Seventh International Coral Reef Symposium, Guam. Vol 1.
44. Westmacott, S., ed. 2000. Management of Bleached and Severely Damaged Coral Reefs. Gland; Cambridge: IUCN.
45. Done, Terence T., and others. 2001. “Scientific Principles for Establishing MPAs to Alleviate Coral Bleaching and
Promote Recovery.” In Coral Bleaching and Marine Protected Areas. Proceedings of the Workshop on Mitigating Coral
Bleaching Impact through MPA Design. Bishop Museum, Honolulu, Hawaii, 29-31 May 2001. Asia Pacific Coastal
Program Report #0102-Pages: 53-59. http://epubs.aims.gov.au/handle/11068/6542.
46. West, J., and R. Salm. 2003. Resistance and Resilience to Coral Bleaching: Implications for Coral Reef Conservation
and Management.” Conservation Biology. 17.4: 956–67.
47. Hansen, L. 2003. Buying Time: A User’s Manual for Building Resistance and Resilience to Climate Change in Natural
Systems. Chapter 6: Tropical Marine. WWF Climate Change Program.
48. Obura, D. 2005. Resilience and Climate Change: Lessons from Coral Reefs and Bleaching in the Western Indian
Ocean.” Estuarine, Coastal and Shelf Science 63.3: 353–72.
49. McClanahan, T., M. Ateweberhan, C. Muhando, J. Maina, and M. Mohammed. 2007. Effects of Climate and Seawater
Temperature Variation on Coral Bleaching and Mortality.” Ecological Monographs. 77.4: 503–525.
50. Keller, B., D. Gleason, E. McLeod, C. Woodley, S. Airamé, B. Causey, A. Friedlander, R. Grober-Dunsmore,
J. Johnson, S. Miller, R. Steneck. 2009. Climate Change, Coral Reef Ecosystems, and Management Options for Marine
Protected Areas. Environmental Management. 44.6: 1069–88.
51. Baskett, M., R. Nisbet, C. Kappel, P. Mumby, and S. Gaines. 2010. Conservation Management Approaches to
Protecting the Capacity for Corals to Respond to Climate Change: A Theoretical Comparison. Global Change
Biology 16. 4: 1229–46.
52. Ban, N., V. Adams, G. Almany, S. Ban, J. Cinner, L. McCook, M. Mills, R. Pressey, and A. White. 2011. Designing,
Implementing and Managing Marine Protected Areas: Emerging Trends and Opportunities for Coral Reef Nations.
Journal of Experimental Marine Biology and Ecology. 408.1.2: 21–31.
53. 53. Maynard, J., S. McKagan, S. Johnson, P. Houk, G. Ahmadia, R. van Hooidonk, L. Harriman, and E. McLeod.
2012. Coral Reef Resilience to Climate Change in Saipan, CNMI; Field-Based Assessments and Implications for
Vulnerability and Future Management. Prepared for CNMI DEQ and NOAA as part of the Northern Mariana Islands
Coral Reef Initiative with The Nature Conservancy, Pacific Marine Resources Institute and the CNMI Division of Fish
and Wildlife as collaborating agencies.
54. Magris, R., R. Pressey, R. Weeks, and N. Ban. 2014. Integrating Connectivity and Climate Change into Marine
Conservation Planning. Biological Conservation. 170: 207–21.
55. Magris, R., S. Heron, and R. Pressey. 2015. Conservation Planning for Coral Reefs Accounting for Climate Warming
Disturbances. Edited by Bayden D Russell. PLOS ONE. 10.11: e0140828.
56. Pandolfi, J. Ecology: Deep and Complex Ways to Survive Bleaching. 2015. Nature. 518.7537: 43–44.
57. Edwards, H., I. Elliott, M. Eakin, A. Irikawa, J. Madin, M. Mcfield, J. Morgan, R. Woesik, and P. Mumby. 2011.
How Much Time Can Herbivore Protection Buy for Coral Reefs under Realistic Regimes of Hurricanes and Coral
Bleaching?: CORAL AND CLIMATE CHANGE.” Global Change Biology . 17.6: 2033–48.
43
58. Game, E., M. Bode, E. McDonald-Madden, H. Grantham, and H. Possingham. 2009. Dynamic Marine Protected
Areas Can Improve the Resilience of Coral Reef Systems: Dynamic MPAs in Coral Reef Systems.” Ecology Letters.
12.12: 1336–46.
59. McClanahan, T. 2014. Recovery of Functional Groups and Trophic Relationships in Tropical Fisheries Closures. Marine
Ecology Progress Series. 497: 13–23.
60. Williams I., D. White, R. Sparks, K. Lino, J. Zamzow, E. Kelly, H. Ramey. 2016. Responses of herbivorous fishes and
benthos to 6 years of protection at the Kahekili Herbivore Fisheries Management Area, Maui. PLoS ONE.
11, e0159100. (doi:10.1371/journal.pone.0159100).
61. Bonaldo, R., A. Hoey, and D. Bellwood. 2014. The Ecosystem Roles of Parrotfishes on Tropical Reefs. Oceanography
and Marine Biology: An Annual Review. 52: 81–132.
62. Adam, T., D. Burkepile, B. Ruttenberg, and M. Paddack. 2015. Herbivory and the Resilience of Caribbean Coral
Reefs: Knowledge Gaps and Implications for Management. Marine Ecology Progress Series. 520: 1–20.
63. Cernohorsky, N., T. McClanahan, I. Babu, and M. Horsak. 2015. Small Herbivores Suppress Algal Accumulation on
Agatti Atoll, Indian Ocean. Coral Reefs. 34: 1023–35.
64. Mumby, P., N. Wolff, Y. Bozec, I. Chollett, and P. Halloran. 2014. Operationalizing the Resilience of Coral Reefs in an
Era of Climate Change: Mapping Resilience. Conservation Letters. 7.3: 176–87.
65. Bozec, Y., S. O’Farrell, J. Bruggemann, B. Luckhurst, and P. Mumby. 2016. Tradeoffs between Fisheries Harvest and
the Resilience of Coral Reefs. Proceedings of the National Academy of Sciences.
66. Aswani, S., P. Mumby, A. Baker, P. Christie, L. McCook, R. Steneck, and R. Richmond. 2015. Scientific Frontiers in
the Management of Coral Reefs. Frontiers in Marine Science 2.
67. Hansen, L. 2003. Buying Time: A User’s Manual for Building Resistance and Resilience to Climate Change in Natural
Systems. Chapter 6: Tropical Marine. WWF Climate Change Program.
68. Arnold, S., R. Steneck, and P. Mumby. 2010. Running the Gauntlet: Inhibitory Effects of Algal Turfs on the Processes
of Coral Recruitment.” Marine Ecology Progress Series. 414: 91–105.
69. McLeod, E., R. Salm, A. Green, and J. Almany. 2009. Designing Marine Protected Area Networks to Address the
Impacts of Climate Change. Frontiers in Ecology and the Environment. 7.7: 362–70.
70. Amar, K. and B. Rinkevich. 2007. A Floating Mid-Water Coral Nursery as Larval Dispersion Hub: Testing an Idea.
Marine Biology. 151.2: 713–18.
71. Raymundo, L., A. Maypa, E. Gomez, and P. Cadiz. 2007. Can Dynamite-Blasted Reefs Recover? A Novel, Low-Tech
Approach to Stimulating Natural Recovery in Fish and Coral Populations. Marine Pollution Bulletin. 54.7: 1009–19.
72. Gomez, E., P. Cabaitan, H. Yap, and R. Dizon. 2014. Can Coral Cover Be Restored in the Absence of Natural
Recruitment and Reef Recovery?: Restoring Coral Cover. Restoration Ecology. 22.2: 142–50.
73. Lirman, D., T. Thyberg, J. Herlan, C. Hill, C. Young-Lahiff, S. Schopmeyer, B. Huntington, R. Santos, and C. Drury.
2010. Propagation of the Threatened Staghorn Coral Acropora Cervicornis: Methods to Minimize the Impacts of
Fragment Collection and Maximize Production. Coral Reefs. 29.3: 729–35.
74. Diaz-Pulido, G., and L. McCook. 2002. The Fate of Bleached Corals: Patterns and Dynamics of Algal Recruitment.
Marine Ecology Progress Series. 232: 115–128.
75. Roff, G., S. Bejarano, Y. Bozec, M. Nugues, R. Steneck, and P. Mumby. 2014. Porites and the Phoenix Effect:
Unprecedented Recovery after a Mass Coral Bleaching Event at Rangiroa Atoll, French Polynesia.
Marine Biology. 161.6: 1385–93.
76. Beeden, R., J. Maynard, J. Johnson, J. Dryden, S. Kininmonth, and P. Marshall. 2014. No-Anchoring Areas Reduce
Coral Damage in an Effort to Build Resilience in Keppel Bay, Southern Great Barrier Reef. Australasian Journal of
Environmental Management. 21.3: 311–19.
77. Yeemin, T., V. Mantachitra, S. Plathong, P. Nuclear, W. Klinthong, and M. Sutthacheep. 2012. Impacts of Coral
Bleaching, Recovery and Management in Thailand. In Proc. 12th Int. Coral Reef Symp., Cairns, Australia.
http://www.icrs2012.com/proceedings/manuscripts/ICRS2012_17D_5.pdf.
44
78. Tun, K., L. Ming Chou, J. Low, T. Yeemin, N. Phongsuwan, N. Setiasih, J. Wilson, et al. 2010. 1.1 A Regional Overview
on the 2010 Coral Bleaching Event in Southeast Asia. Global Coral Reef Monitoring Network, Ministry of the
Environment, Japan.
79. GBRMPA (Great Barrier Reef Marine Park Authority). 2008. Aquarium Collectors’ World First Climate Change Initiative.
SeaRead.
80. Bonin, Mary C., H. Harrison, D. Williamson, A. Frisch, P. Saenz-Agudelo, M. Berumen, and G. Jones. 2016.
The Role of Marine Reserves in the Replenishment of a Locally Impacted Population of Anemonefish on the Great
Barrier Reef. Molecular Ecology. 25.2: 487–99. doi:10.1111/mec.13484.
81. Mbije, N., E. Spanier, and B. Rinkevich. 2013. A First Endeavour in Restoring Denuded, Post-Bleached Reefs in
Tanzania. Estuarine, Coastal and Shelf Science. 128 (August): 41–51. doi:10.1016/j.ecss.2013.04.021.
82. McClanahan, T., J. Maina, C. Starger, P. Herron-Perez, and E. Dusek. 2005. Detriments to Post-Bleaching Recovery of
Corals. Coral Reefs. 24.2: 230–46. doi:10.1007/s00338-004-0471-1.
83. Armstrong, J. S. 2001. Combining forecasts. Pages 417–440 in J. S. Armstrong, editor. Principles of forecasting: a
handbook for researchers and practitioners. Kluwer Academic Publishers, Norwell, Massachusetts.
84. Beverton, R., & S. Holt. 1957. On the dynamics of exploited fish populations, Fishery Investigations Series II, Vol. XIX,
Ministry of Agriculture. Fisheries and Food. 1: 957.
85. Polacheck, T. 1990. Year around closed areas as a management tool. Natural Resource Modeling, 4.3, 327-354.
86. DeMartini, E. 1993. Modeling the potential of fishery reserves for managing Pacific coral-reef fishes. Fishery Bulletin,
91.3: 414-427.
87. McClanahan, T., & B. Kaunda-Arara. 1996. Fishery recovery in a coral-reef marine park and its effect on the adjacent
fishery. Conservation Biology, 10.4: 1187-1199.
88. Nowlis, J., & C. Roberts. 1999. Fisheries benefits and optimal design of marine reserves. Fishery Bulletin, 97.3:
604-616.
89. Roberts, C., J. Bohnsack, F. Gell, J. Hawkins, & R. Goodridge. 2001. Effects of marine reserves on adjacent fisheries.
Science: 294.5548: 1920-1923.
90. Russ, G., & C. Alcala. 2004. Marine reserves: long-term protection is required for full recovery of predatory fish
populations. Oecologia. 138.4: 622-627.
91. Abesamis, R., & G. Russ. 2005. Density–dependent spillover from a marine reserve: Long–term evidence. Ecological
applications. 15.5: 1798-1812.
92. Graham, N., T. Ainsworth, A. Baird, N. Ban, L. Bay, J. Cinner, D. De Freitas, et al. 2011. From Microbes to People:
Tractable Benefits of No-Take Areas for Coral Reefs. Oceanography and Marine Biology-an Annual Review. 49: 105.
93. Bohnsack, J. A. 1998. Application of marine reserves to reef fisheries management. Austral Ecology. 23.3: 298-304.
94. Roberts, C., J. Hawkins, & F. Gell. 2005. The role of marine reserves in achieving sustainable fisheries. Philosophical
Transactions of the Royal Society of London B: Biological Sciences. 360.1453: 123-132.
95. Mumby, P., A. Harborne, J. Williams, C. Kappel, D. Brumbaugh, F. Micheli, K. Holmes, C. Dahlgren, C., and
P. Blackwell. 2007. Trophic Cascade Facilitates Coral Recruitment in a Marine Reserve. Proceedings of the National
Academy of Sciences. 104.20: 8362–8367.
96. Friedlander, A., E. Brown, and M. Monaco. 2007. Defining Reef Fish Habitat Utilization Patterns in Hawaii:
Comparisons between Marine Protected Areas and Areas Open to Fishing. Marine Ecology Progress Series. 351
(December): 221–33. doi:10.3354/meps07112.
97. Stockwell, B., C. Jadloc, R. Abesamis, A. Alcala, and G. Russ. 2009. Trophic and Benthic Responses to No-Take
Marine Reserve Protection in the Philippines. Marine Ecology Progress Series. 389 (September): 1–15.
doi:10.3354/meps08150.
98. Selig, E.., and J. Bruno. 2010. A Global Analysis of the Effectiveness of Marine Protected Areas in Preventing Coral
Loss. PLoS One. 5.2: e9278.
99. Graham, N., D. Bellwood, J. Cinner, T. Hughes, A. Norström, and M. Nyström. 2013. Managing Resilience to Reverse
Phase Shifts in Coral Reefs.” Frontiers in Ecology and the Environment. 11.10: 541–48.
45
100. Heenan, A., R. Pomeroy, J. Bell, P. Munday, W. Cheung, C. Logan, R. Brainard, et al. 2015. A Climate-Informed,
Ecosystem Approach to Fisheries Management. Marine Policy. 57 (July): 182–92.
doi:10.1016/j.marpol.2015.03.018.
101. Williams G., Gove J., Eynaud Y., Zgliczynski B., Sandin S.. 2015. Local human impacts decouple natural biophysical
relationships on Pacific coral reefs. Ecography. 38: 751–761. (doi:10.1111/ecog.01353)
102. Williams I., Baum J., Heenan A, Hanson K., Nadon M., Brainard R. 2015. Human, oceanographic and habitat
drivers of central and western Pacific coral reef fish assemblages. PLoS ONE. 10, e0120516.
(doi:10.1371/journal.pone.0120516).
103. Williams S., Chollett I., Roff G., Corte´s J., Dryden C., Mumby P. 2015. Hierarchical spatial patterns in Caribbean reef
benthic assemblages. J. Biogeogr. 42: 1327–1335. (doi:10.1111/jbi.12509).
104. Howard, K., J. Claisse, T. Clark, K. Boyle, and J. Parrish. 2013. Home Range and Movement Patterns of the Redlip
Parrotfish (Scarus Rubroviolaceus) in Hawaii. Marine Biology 160.7: 1583–95. doi:10.1007/s00227-013-2211-y.
105. McCook, L., J. Jompa, and G. Diaz-Pulido. 2001. Competition between Corals and Algae on Coral Reefs: A Review of
Evidence and Mechanisms. Coral Reefs.19. 4: 400–417. doi:10.1007/s003380000129.
106. Knowlton, N. 2004. Multiple “stable” states and the conservation of marine ecosystems. Progress in Oceanography.
60.2: 387-396.
107. Bellwood D., C. Fulton. 2008. Sediment-mediated suppression of herbivory on coral reefs: decreasing resilience to
rising sea-levels and climate change? Limnol. Oceanogr. 53. 2695–2701. doi:10.4319/lo.2008.53.6.2695.
108. Mangi, S., & C. Roberts, C. M. 2006. Quantifying the environmental impacts of artisanal fishing gear on Kenya’s
coral reef ecosystems. Marine pollution bulletin. 52. 12: 1646-1660.
109. McClanahan, T., and J. Cinner. 2008. A Framework for Adaptive Gear and Ecosystem-Based Management in the
Artisanal Coral Reef Fishery of Papua New Guinea. Aquatic Conservation: Marine and Freshwater Ecosystems.
18.5: 493–507. doi:10.1002/aqc.874.
110. Cinner, J., T. McClanahan, N. Graham, M. Pratchett, S. Wilson, and J. Raina. 2009. Gear-Based
Fisheries Management as a Potential Adaptive Response to Climate Change and Coral Mortality. Journal of Applied
Ecology. 46.3: 724–32. doi:10.1111/j.1365-2664.2009.01648.x.
111. Puleloa, W. Moloka‘i Island Gill-net Project. 2012. Department of Land and Natural Resources.
112. Meyer, Carl G. 2007. The Impacts of Spear and Other Recreational Fishers on a Small Permanent Marine Protected
Area and Adjacent Pulse Fished Area. Fisheries Research. 84.3: 301–7. doi:10.1016/j.fishres.2006.11.004.
113. Lindfield, S., J. McIlwain, and E. Harvey. 2014. Depth Refuge and the Impacts of SCUBA Spearfishing on Coral Reef
Fishes. PloS One. 9.3: e92628.
114. Stoffle, B., S. Allen. 2012. The Sociocultural Importance of Spearfishing in Hawai‘i. US Department of
Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Pacific Islands
Fisheries Science Center.
115. Bellwood, D., A. Baird, M. Depczynski, A. González-Cabello, A. Hoey, C. Lefèvre, and J. Tanner. 2012. Oecologia.
170: 567–573.
116. Edwards, C., A. Friedlander, A. Green, M. Hardt, E. Sala, H. Sweatman, & J. Smith. 2014. Global assessment of the
status of coral reef herbivorous fishes: evidence for fishing effects. Proceedings of the Royal Society of London B:
Biological Sciences. 281.1774: 20131835.
117. Heenan, A., A. Hoey, G. Williams, and I. Williams. 2016. Natural Bounds on Herbivorous Coral Reef Fishes.
Proceedings of the Royal Society B: Biological Sciences 283.1843: 20161716. doi:10.1098/rspb.2016.1716.
118. Nadon, M. O. 2017. Stock assessment of the coral reef fishes of Hawaii, 2016. U.S. Dep. Commer., NOAA Tech.
Memo., NOAA-TM-NMFS-PIFSC-60, 212 p. doi:10.7289/V5/TM-PIFSC-60.
119. Cox, C., C. Jones, J. Wares, K. Castillo, M. McField, and J. Bruno. 2013. Genetic Testing Reveals Some Mislabeling
but General Compliance with a Ban on Herbivorous Fish Harvesting in Belize: Genetic Testing on Coral Reef Fish in
Belize. Conservation Letters 6.2: 132–40. doi:10.1111/j.1755-263X.2012.00286.x.
46
120. O’Farrell, S., B. Luckhurst, S. Box, and P. Mumby. 2016. Parrotfish Sex Ratios Recover Rapidly in Bermuda
Following a Fishing Ban. Coral Reefs. 35.2: 421–25. doi:10.1007/s00338-015-1389-5.
121. Carassou, L., M. Léopold, N. Guillemot, L. Wantiez, and N. Kulbicki. 2013. Does Herbivorous Fish Protection Really
Improve Coral Reef Resilience? A Case Study from New Caledonia (South Pacific). Edited by Richard KF. Unsworth.
PLoS ONE. 8.4: e60564. doi:10.1371/journal.pone.0060564.
122. O’Farrell, S., A. Harborne, Y. Bozec, B. Luckhurst, and P. Mumby. 2015. Protection of Functionally Important
Parrotfishes Increases Their Biomass but Fails to Deliver Enhanced Recruitment. Marine Ecology Progress Series.
522 (March): 245–54. doi:10.3354/meps11134.
123. Mumby, P. 2006. Fishing, Trophic Cascades, and the Process of Grazing on Coral Reefs. Science. 311.
5757: 98–101. doi:10.1126/science.1121129.
124. Ledlie, M., N. Graham, J. Bythell, S. Wilson, S. Jennings, N. Polunin, and J. Hardcastle. 2007. Phase Shifts and the
Role of Herbivory in the Resilience of Coral Reefs. Coral Reefs. 26. 3: 641–53. doi:10.1007/s00338-007-0230-1.
125. Jayewardene, D. 2009. A Factorial Experiment Quantifying the Influence of Parrotfish Density and Size on Algal
Reduction on Hawaiian Coral Reefs. Journal of Experimental Marine Biology and Ecology. 375.1–2: 64–69.
doi:10.1016/j.jembe.2009.05.006.
126. Jouffray, J., M. Nystrom, A. Norstrom, I. Williams, L. Wedding, J. Kittinger, and G. Williams. 2014. Identifying
Multiple Coral Reef Regimes and Their Drivers across the Hawaiian Archipelago. Philosophical Transactions of the
Royal Society B: Biological Sciences. 370.1659: 20130268–20130268. doi:10.1098/rstb.2013.0268.
127. DeMartini, E., and K. Howard. 2016. Comparisons of Body Sizes at Sexual Maturity and at Sex Change in the
Parrotfishes of Hawai‘i: Input Needed for Management Regulations and Stock Assessments: Comparative Maturation
of Parrotfishes. Journal of Fish Biology. 88.2: 523–41. doi:10.1111/jfb.12831.
128. Ong, L., and K. Holland. 2010. Bioerosion of Coral Reefs by Two Hawaiian Parrotfishes: Species, Size Differences and
Fishery Implications. Marine Biology. 157.6: 1313–23. doi:10.1007/s00227-010-1411-y.
129. Gil, M., S. Goldenberg, A. Bach, S. Mills, and J. Claudet. 2016. Interactive Effects of Three Pervasive Marine
Stressors in a Post-Disturbance Coral Reef. Coral Reefs. 35.4: 1281–93. doi:10.1007/s00338-016-1489-x.
130. Richmond, R., T. Rongo, Y. Golbuu, S. Victor, N. Idechong, G. Davis, W. Kostka, L. Neth, M. Hamnett, and
E. Wolanski. 2007. Watersheds and Coral Reefs: Conservation Science, Policy, and Implementation. BioScience.
57.7: 598–607.
131. Chu, Z., S. Zhai, X. Lu, J. Liu, J. Xu, K. Xu, 2009. A quantitative assessment of human impacts on decrease in
sediment flux from major Chinese rivers entering the western Pacific Ocean. Geophys. Res. Lett. 36: 1–5.
132. Kroon, F., B. Schaffelke, and R. Bartley. 2014. Informing Policy to Protect Coastal Coral Reefs: Insight from a Global
Review of Reducing Agricultural Pollution to Coastal Ecosystems. Marine Pollution Bulletin. 85.1: 33–41.
doi:10.1016/j.marpolbul.2014.06.003.
133. Rodgers, K., M. Kido, P. Jokiel, T. Edmonds, and E. Brown. 2012. Use of Integrated Landscape Indicators to Evaluate
the Health of Linked Watersheds and Coral Reef Environments in the Hawaiian Islands. Environmental Management.
50.1: 21–30. doi:10.1007/s00267-012-9867-9.
134. Smith, S., W. Kimmerer, E. Laws, R. Brock, T. Walsh. 1981. Kaneohe Bay sewage diversion experiment–perspectives
on ecosystem responses to nutritional perturbation. Pac. Sci. 35: 279–402.
135. Rinkevich, Baruch. 2005. Conservation of Coral Reefs through Active Restoration Measures: Recent Approaches and
Last Decade Progress. Environmental Science & Technology. 39.12: 4333–42. doi:10.1021/es0482583.
136. Rinkevich, B. 2006. The Coral Gardening Concept and the Use of Underwater Nurseries: Lessons Learned from
Silvics and Silviculture. In Coral Reef Restoration Handbook, edited by William F. Precht.
137. Rinkevich, B. 2008. Management of Coral Reefs: We Have Gone Wrong When Neglecting Active Reef Restoration.
Marine Pollution Bulletin. 56. 11: 1821–24. doi:10.1016/j.marpolbul.2008.08.014.
138. Van Oppen, M., P. Bongaerts, J. Underwood, L. Peplow, and T. Cooper. 2011. The Role of Deep Reefs in Shallow Reef
Recovery: An Assessment of Vertical Connectivity in a Brooding Coral from West and East Australia: vertical
connectivity in a brooding coral. Molecular Ecology. 20.8: 1647–60. doi:10.1111/j.1365-294X.2011.05050.x.
47
139. D’angelo, C., B. Hume, J. Burt, E. Smith, E. Achterberg, and J. Wiedenmann. 2015. Local Adaptation Constrains the
Distribution Potential of Heat-Tolerant Symbiodinium from the Persian/Arabian Gulf. The ISME Journal.
9.12: 2551–2560.
140. Cremieux, L., A. Bischoff, H. Muller-Scharer, and T. Steinger. 2010. Gene Flow from Foreign Provenances into Local
Plant Populations: Fitness Consequences and Implications for Biodiversity Restoration. American Journal of Botany.
97.1: 94–100. doi:10.3732/ajb.0900103.
141. Pollnac, R., P. Christie, J. Cinner, T. Dalton, T. Daw, G. Forrester, et al., Marine reserves as linked social–ecological
systems. 2010. Proc.Natl.Acad.Sci. 107: 18262–18265. http://dx.doi.org/10.1073/pnas.0908266107.
142. Edgar, G., R. Stuart-Smith, T. Willis, S. Kininmonth, S. Baker, S. Banks, et al. 2014. Global conservation outcomes
depend on marine protected areas with five key features, Nature. 50: 216–220.
http://dx.doi.org/10.1038/ nature13022.
143. DLNR. 2015. North Maui Community Fisheries Enforcement Unit Update: Educate, Outreach, and Enforcement
Leads to Healthier Resources. http://dlnr.hawaii.gov/blog/2016/08/25/nr16-166/.
144. Crawford, B., A. Siahainenia, C. Rotinsulu, and A. Sukmara. 2004. Compliance and enforcement of community-
based coastal resource management regulations in North Sulawesi, Indonesia. Coastal Management. 32.1: 39-50.
145. McClanahan, T., M. Marnane, J. Cinner, and W. Kiene. 2006. A Comparison of Marine Protected Areas and Alterna-
tive Approaches to Coral-Reef Management. Current Biology. 16.14: 1408–13. doi:10.1016/j.cub.2006.05.062.
146. Haisfield, K., H. Fox, S. Yen, S. Mangubhai, & P. Mous. 2010. An ounce of prevention: cost-effectiveness of coral reef
rehabilitation relative to enforcement. Conservation Letters. 3.4: 243-250.
147. Kaplan, K., G. Ahmadia, H. Fox, L. Glew, E. Pomeranz, and P. Sullivan. 2015. Linking Ecological Condition to
Enforcement of Marine Protected Area Regulations in the Greater Caribbean Region. Marine Policy . 62 (December):
186–95. doi:10.1016/j.marpol.2015.09.018.
