www.acs.org/middleschoolchemistry 1 ©2023 American Chemical Society
Chapter 4, Lesson 4: Energy Levels, Electrons, and Covalent Bonding
Key Concepts
• The electrons on the outermost energy level of the atom are called valence
electrons.
• The valence electrons are involved in bonding one atom to another.
• The attraction of each atom’s nucleus for the valence electrons of the other
atom pulls the atoms together.
• As the attractions bring the atoms together, electrons from each atom are
attracted to the nucleus of both atoms, which “share” the electrons.
• The sharing of electrons between atoms is called a covalent bond, which
holds the atoms together as a molecule.
• A covalent bond happens if the attractions are strong enough in both
atoms and if each atom has room for an electron in its outer energy level.
• Atoms will covalently bond until their outer energy level is full.
• Atoms covalently bonded as a molecule are more stable than they were as
separate atoms.
Summary
Students will look at animations and refer to the energy level models they have
been using to make drawings of the process of covalent bonding. Students will
consider why atoms bond to form molecules like H
2
(hydrogen), H
2
O (water), O
2
(oxygen), CH
4
(methane), and CO
2
(carbon dioxide).
Objective
Students will be able to explain that attraction between the protons and electrons of
two atoms cause them to bond. Students will be able to draw a model of the
covalent bonds between the atoms in H
2
(hydrogen), H
2
O (water), O
2
(oxygen), CH
4
(methane), and CO
2
(carbon dioxide).
Evaluation
The activity sheet will serve as the “Evaluate” component of each 5-E lesson plan.
The activity sheets are formative assessments of student progress and
understanding.
Safety
Be sure you and the students wear properly fitting goggles.
About this Lesson
This lesson will probably take more than one class period.

www.acs.org/middleschoolchemistry 2 ©2023 American Chemical Society
Materials for Each Group
• 9-volt battery
• 2 wires with alligator clips on both ends
• 2 pencils sharpened at both ends
• Water
• Clear plastic cup
• Epsom Salt (magnesium sulfate)
• Tape
ENGAGE
1.
Show an animation to introduce the process of covalent bonding.
Introduce the question students will investigate in this lesson:
• If atoms have an equal number of protons and electrons, why do
atoms bond to other atoms? Why don’t they just stay separate?
Begin to answer this question by using hydrogen as an example.
Project the animation Covalent bond in hydrogen.
www.acs.org/middleschoolchemistry/simulations/chapter4/lesson4.html
Make sure students see that each hydrogen atom
has 1 proton and 1 electron. Remind students that
the electron and its own proton are attracted to
each other. Explain that if the atoms get close
enough to each other, the electron from each hydrogen atom feels the
attraction from the proton of the other hydrogen atom (shown by the double-
headed arrow).
Point out to students that the attractions are not strong enough to pull the
electron completely away from its own proton. But the attractions are strong
enough to pull the two atoms close enough together so that the electrons feel
the attraction from both protons and are shared by both atoms.
At the end of the animation, explain that the individual hydrogen atoms have
now bonded to become the molecule H
2
. This type of bond is called a covalent
bond. In a covalent bond, electrons from each atom are attracted or “shared”
by both atoms.
Read more about bonding
in Teacher Background.

www.acs.org/middleschoolchemistry 3 ©2023 American Chemical Society
EXPLAIN
2.
Discuss the conditions needed for covalent bonding and the stable molecule
that is formed.
Project the image Covalent bond in hydrogen.
www.acs.org/middleschoolchemistry/simulations/chapter4/lesson4.html
Note: This model of covalent bonding for the hydrogen molecule (H
2
) starts with
2 individual hydrogen atoms. In reality, hydrogen atoms are never separate to
start with. They are always bonded with something else. To simplify the process,
this model does not show the hydrogen atoms breaking their bonds from other
atoms. It only focuses on the process of forming covalent bonds between two
hydrogen atoms.
Tell students that there are two main reasons why two hydrogen atoms bond
together to make one hydrogen molecule:
• There needs to be a strong enough attraction between the electrons of
each atom for the protons of the other atom.
• There needs to be room in the outer energy level of both atoms.
Two hydrogen atoms are near each other.
When two hydrogen atoms get close enough to
each other, their electrons are attracted to the
protons in the other atom.
Because there is both a strong enough attraction
between atoms and room in the outer energy level of
both atoms, the atoms share electrons. This is a
covalent bond.
www.acs.org/middleschoolchemistry 4 ©2023 American Chemical Society
Once bonded, the hydrogen molecule is more stable than the individual hydrogen
atoms. Explain to students that by being part of a covalent bond, the electron
from each hydrogen atom gets to be near two protons instead of only the one
proton it started with. Since the electrons are closer to more protons, the
molecule of two bonded hydrogen atoms is more stable than the two individual
unbonded hydrogen atoms.
This is why it is very rare to find a hydrogen atom that is not bonded to other
atoms. Hydrogen atoms bond with other hydrogen atoms to make hydrogen gas
(H
2
). Or they can bond with other atoms like oxygen to make water (H
2
O) or
carbon to make methane (CH
4
) or many other atoms.
3. Show students that when two hydrogen atoms bond together, the outer
energy level becomes full.
Have students look at their Periodic table of energy levels for elements 1–20
distributed in lesson 3.
Explain that the two electrons in the hydrogen molecule (H
2
) can be thought of as
“belonging” to each atom. This means that each hydrogen atom now has two
electrons in its first energy level. The first energy level in the outer energy level
for hydrogen and can only accommodate or “hold” two electrons. Atoms will
continue to covalently bond until their outer energy levels are full. At this point,
additional atoms will not covalently bond to the atoms in the H
2
molecule.
4. Have students describe covalent bonding in a hydrogen molecule on their
activity sheet and then review their answers.
Give each student an activity sheet.
Have students write a short caption under each picture to describe the process of
covalent bonding and answer the first three questions. The rest of the activity
sheet will either be completed as a class, in groups, or individually, depending on
your instructions.
Ask students:
• What did you write for the second and third pictures of covalent
bonding?
Center drawing: When two hydrogen atoms come close enough, their
electrons are attracted to the proton of the other atom.
Last drawing: This brings the atoms close enough together that they share
electrons.

www.acs.org/middleschoolchemistry 5 ©2023 American Chemical Society
• What are two conditions atoms must have in order to form covalent
bonds with one another?
There is a strong enough attraction between atoms and there is room
for electrons in the outer energy level of both atoms.
• Why is a hydrogen molecule (H
2
) more stable than two individual
hydrogen atoms?
In the hydrogen molecule, the electrons from each atom are able to be
near two protons instead of only the one proton it started with.
Whenever negative electrons are near additional positive protons, the
arrangement is more stable.
• Why doesn’t a third hydrogen atom join the H
2
molecule to make H
3
?
When two hydrogen atoms share their electrons with each other, their outer
energy levels are full.
You could explain to students that when the outer energy levels are
full, sharing electrons with another atom would not happen for two
main reasons:
1. An electron from a new atom would have to join an atom in the
H
2
molecule on the next energy level, further from the nucleus
where it would not feel a strong enough attraction.
2. An electron from an atom already in the H
2
molecule and close to the
nucleus would need to move further away to share with the new atom.
Both possibilities would make the molecule less stable and therefore
would not happen.
5.
Discuss the process of covalent bonding in a water molecule.
Project the animation Covalent bond in water.
www.acs.org/middleschoolchemistry/simulations/chapter4/lesson4.html
Before hitting the “play” button, point out the oxygen atom and the two hydrogen
atoms. Ask students:
• Is there anything that might attract these atoms to one another?
Students should suggest that the electrons from each atom are
attracted to the pro- tons of the other atoms.
Play the animation to show the attraction between the protons of oxygen for
the electron from each of the hydrogen atoms, the attraction of the proton
from the hydrogen atoms for the electrons of oxygen, and the atoms coming
together.

www.acs.org/middleschoolchemistry 6 ©2023 American Chemical Society
Explain that the electrons are shared by the oxygen and hydrogen atoms
forming a covalent bond. These bonds hold the oxygen and hydrogen atoms
together and form the H
2
O molecule. The reason why the atoms are able to
bond is that the attractions are strong enough in both directions and there
is room for the electrons on the outer energy level of the atoms.
The electron from each hydrogen atom and the electrons from the oxygen
atom get to be near more protons when the atoms are bonded together as a
molecule than when they are separated as individual atoms. This makes the
molecule of bonded oxygen and hydrogen atoms more stable than the
individual separated atoms.
Explain to students that the two electrons in the bond between the hydrogen
atom and the oxygen atom can be thought of as “belonging” to each atom.
This gives each hydrogen atom two electrons in its outer energy level, which
is full. It also gives oxygen 8 electrons in its out- er energy level, which is also
full.
Project the image Covalent bond in water.
www.acs.org/middleschoolchemistry/simulations/chapter4/lesson4.html
Review with students the process of covalent bonding covered in the animation.
6.
Have students describe covalent bonding in a water molecule on
their activity sheet.
Have students write a short caption beside each picture to describe the
process of covalent bonding in the water molecule.
Two hydrogen atoms and one oxygen atom are near
each other.
Because there is both a strong enough attraction
between atoms and room for electrons in the outer
level of the atoms, they share electrons. This forms a
covalent bond.
When two hydrogen atoms come close enough to an
oxygen atom, their electrons are attracted to the
proton of the other atom.

www.acs.org/middleschoolchemistry 7 ©2023 American Chemical Society
Note: This model of covalent bonding for a water molecule starts with 2
individual hydrogen atoms and 1 oxygen atom. In reality, these atoms are
never separate to start with. They are always bonded with something else. To
simplify the process, this model does not show the hydrogen and oxygen
atoms breaking their bonds from other atoms. It only focuses on the process of
forming covalent bonds to make water.
Ask students:
• Why can’t a third hydrogen atom join the water molecule (H
2
O) to
make H
3
O?
Once the outer energy levels are full, sharing electrons with another
atom would not happen for two main reasons: An electron from a new
atom would have to join an atom in the H
2
O molecule on the next
energy level, further from the nucleus where it would not feel a strong
enough attraction. An electron from an atom already in the H
2
O
molecule and close to the nucleus would need to move further away to
share with the new atom. Both of these possibilities would make the
molecule less stable and would not happen.
EXPLORE
7.
Have students use electricity to form oxygen and hydrogen gas from water.
Tell students that electrical energy causes electrons and atoms from water
molecules to rearrange and produce hydrogen atoms and oxygen atoms. Two
hydrogen atoms bond to form hydrogen gas (H
2
) and two oxygen atoms bond
to form oxygen gas (O
2
).
Note: It is true that in the electrolysis of water, oxygen and hydrogen atoms
from water molecules (H
2
O) eventually become hydrogen gas (H
2
) and
oxygen gas (O
2
). But this is a multi-step process and not a simple breaking of
the covalent bonds in water and immediately reforming new bonds to make
the gases. There are several intermediate steps.
You may choose to do this activity as a demonstration or show the video
Electrolysis.
www.acs.org/middleschoolchemistry/simulations/chapter4/lesson4.html

www.acs.org/middleschoolchemistry 8 ©2023 American Chemical Society
Question to investigate
What is produced when electricity is put into water?
Materials for each group
• 9-volt battery
• 2 wires with alligator clips on both ends
• 2 pencils sharpened at both ends
• Water
• Epsom salt (magnesium sulfate)
• Clear plastic cup
• Tape
Procedure
1. Place a battery between 2 pencils. Be sure that the battery is more than half-
way up.
2. With the help of a partner, wrap tape around the
pencils and battery as shown.
3. Add water to a clear plastic cup until it is
about ½-full.
4. Add about ½ teaspoon of Epsom salt to the water
and stir until the salt dissolves.
5. Connect one alligator clip to one terminal of the battery.
6. Using the other wire, connect one alligator clip to the other terminal of the
battery.
7. Connect one end of the pencil lead to the alligator clip at the end of one of
the wires.
8. Using the other wire, connect one end of the other pencil lead to the alligator
clip at the end of the wire.
9. Place the ends of the pencil into the water as shown.
Expected results
Bubbles will form and rise initially from one pencil lead. Soon, bubbles will
form and rise from the other. Students should be able to see that there is
more of one gas than the other. The gas that forms the small bubbles that
comes off first is hydrogen. The other gas that forms the larger bubbles and
lags behind a bit is oxygen.
www.acs.org/middleschoolchemistry 9 ©2023 American Chemical Society
Note: There will be bubbling when hydrogen and oxygen gas form on the
pencil leads. Be sure students do not get the misconception that the bubbles
they see mean that the water is boiling. In boiling, the bonds holding the
atoms together in water molecules do not come apart. In the process of
electrolysis, the bonds holding the atoms together do come apart.
8.
Discuss student observations.
Ask students:
• What are the bubbles made out of in the activity?
Hydrogen gas (H
2
) and oxygen gas (O
2
)
• Why was there more hydrogen gas produced than oxygen gas?
Each water molecule breaks into 2 hydrogen atoms and 1 oxygen atom.
Two hydrogen atoms then bond to form hydrogen gas (H
2
) and 2 oxygen
atoms bond to form oxygen gas (O
2
).
Each water molecule has all the atoms needed to make 1 molecule of
hydrogen gas. But with only 1 oxygen atom, a water molecule only has
half of what is needed to make 1 molecule of oxygen gas. So, 2 water
molecules will produce 2 molecules of hydrogen gas but only 1 molecule
of oxygen gas.
EXTEND
9.
Help students understand how atoms combine to form the molecules
of oxygen, methane, and carbon dioxide.
Remind students that in this lesson they looked at the covalent bonds in
hydrogen molecules and in water molecules. Tell them that they will look at
the covalent bonds in three other common substances.
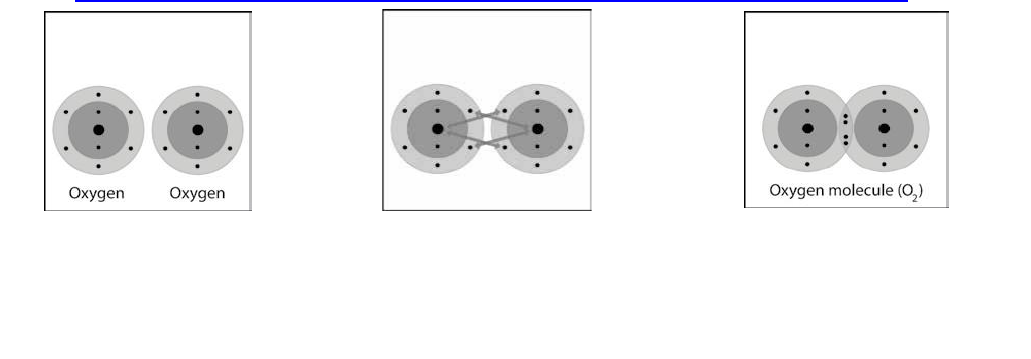
Project the animation Oxygen’s double bond.
www.acs.org/middleschoolchemistry/simulations/chapter4/lesson4.html
Each oxygen atom has 6
valence electrons in its outer
energy level.
When two oxygen atoms get close
to each other, the attractions from
the nucleus of both atoms attract
the outer electrons.
In this case, two electrons from
each atoms are shared. This is
called a double bond.

www.acs.org/middleschoolchemistry 10 ©2023 American Chemical Society
Explain to students that the oxygen molecules that are present in our air are made
up of 2 oxygen atoms. This animation will show what the covalent bond between 2
oxygen atoms is like.
Narrate the animation by pointing out that:
• Each oxygen atom has 6 valence electrons in its outer energy level.
• When the oxygen atoms get close together, the attractions from the
nucleus of both atoms attract the outer electrons from the other atom.
• In this case, 2 electrons from each atom are shared. This is called a double
covalent bond.
Project the image Oxygen’s double bond II.
www.acs.org/middleschoolchemistry/simulations/chapter4/lesson4.html
Review with students the process of covalent bonding covered in the animation.
Project the before and after pictures Covalent bonding of methane.
www.acs.org/middleschoolchemistry/simulations/chapter4/lesson4.html
Ask students:
• Briefly describe the process of covalent bonding between the carbon
and the four hydrogen atoms to make a methane molecule. Be sure to
mention attractions between electrons and protons and the number of
electrons in the outer energy level for the atoms in the final molecule.
Be sure students realize that the protons of each atom attracts the other
atoms electrons, which brings the atoms together. Atoms continue to
bond with other atoms until their outer energy levels are full.

www.acs.org/middleschoolchemistry 11 ©2023 American Chemical Society
Project the before and after pictures Covalent bonding of carbon dioxide gas.
www.acs.org/middleschoolchemistry/simulations/chapter4/lesson4.html
Ask students:
• Briefly describe the process of covalent bonding between the carbon
and the two oxygen atoms to make a carbon dioxide molecule. Be
sure to mention attractions between electrons and protons and the
number of electrons in the outer energy level for the atoms in the
final molecule.
Be sure students realize that the protons of each atom attracts the other
atom’s electrons, which brings the atoms together. Atoms continue to
bond with other atoms until their outer energy levels are full.
