
Franklin’s bumble bee (Bombus franklini)
Species Status Assessment
__________________________________________________
Photo of Bombus franklini by Pete Schroeder
Final Report (Version 1)
June 25, 2018
2
Prepared by Jeff Everett from the Oregon Fish and Wildlife office, Portland, Oregon; with
assistance from Nadine Kanim (Yreka Fish and Wildlife Office), Rebecca Migala (Pacific
Region Office), and Deb Giglio (Pacific Southwest Region Office).
Acknowledgements
We would like to thank the following individuals who provided substantive information and
insights for our analysis: Gene Pierce, United Stated Department of Agriculture, National
Agricultural Statistics Service; Jon Jinings, Oregon Department of Land Conservation and
Development; Stephen Haney and Steve Godwin, Bureau of Land Management; Sheila Colyer
and Bill Shaupp, U.S. Forest Service; Jennifer Syzmanski, Pepper Trail, and Tamara Smith, US
Fish & Wildlife Service; Dr. Robbin Thorp, University of California at Davis; Rich Hatfield,
Xerces Society; Peter Schroeder; Dr. Andony Melathopolous, Oregon State University; Dr. Carol
Ferguson, Southern Oregon University; Robin Fallshear, Califonia Department of Fish and
Wildlife; Dr. Sheila Colla, York University; Sydney Cameron, University of Illinois at Urbana-
Champaign; Sarah Kincaid and Helmuth Rogg, Oregon Department of Agriculture.
3
Executive Summary
This document presents the species status assessment (SSA) for Franklin’s bumble bee (Bombus
franklini), completed to characterize the species’ overall viability. To characterize viability we
use the three conservation biology principles of resiliency, representation, and redundancy. We
identify the species’ ecological requirements for survival and reproduction at the individual,
population, and species levels, and describe risk factors influencing the species’ current and
future condition.
Bombus franklini has the most restricted range of any North American bumble bee, and possibly
the most restricted range of any bumble bee in the world. Historically, B. franklini occupied
portions of Douglas, Jackson and Josephine Counties in southern Oregon, as well as Trinity and
Siskiyou Counties in northern California. Since the late 1990s, B. franklini observations have
declined significantly, and none have been observed since 2006, despite an expanded and
focused survey effort. Very little is known about B. franklini; much of the information presented
in this SSA is inferred from closely-related Bombus species, and we rely heavily on information
from species experts. While the decline of B. franklini observations is contemporaneous with the
decline of other Bombus species, the causal factors behind these declines are poorly understood.
The species has likely been affected by pathogens, pesticides, and the effects of small population
size. The synergistic effects of several stressors to the species have likely exacerbated declines.
Bombus franklini has been found in a wide array of sheltered and exposed habitat types at a
broad elevational range, and the species appears to be a generalist forager. Our certainty
regarding the species’ habitat needs is limited to (1) floral resources for nectaring throughout the
colony cycle, and (2) relatively protected areas for breeding and shelter. The habitat elements
that B. franklini appears to prefer to fulfil those needs are relatively flexible, plentiful, and
widely distributed. Despite this fact, no individuals of the species have been found in any habitat
since 2006, and therefore we conclude that the resiliency of the species has decreased since the
1990s. Further, no current populations of B. franklini, distributed across any level of ecological
conditions or spatial extent, are known to exist, and therefore we conclude that genetic and
ecological representation as well as redundancy have decreased since the 1990s. Due to the lack
of observations of the species since 2006, we did not project anticipated future states of
resiliency, redundancy or representation. Although the failure to detect a species during surveys
is not equivalent to a conclusive demonstration of its absence and may simply reflect the very
low detection probability for rare species, the certain losses in both the number of populations
and their spatial extent render B. franklini vulnerable to extinction even without further external
stressors acting upon the species.
4
Contents
Acknowledgements ....................................................................................................................................... 2
Executive Summary ....................................................................................................................................... 3
1.0 Introduction, Analytical Framework, and Methods ................................................................................ 6
1.1 Introduction ........................................................................................................................................ 6
1.2 Analytical Framework ......................................................................................................................... 7
1.2.1 Resiliency ..................................................................................................................................... 7
1.2.2 Representation ............................................................................................................................. 7
1.2.3 Redundancy ................................................................................................................................. 8
1.3 Methods .............................................................................................................................................. 8
2.0 Species Information .............................................................................................................................. 10
2.1 Background ....................................................................................................................................... 10
2.1.1Taxonomy and Species Description ............................................................................................ 10
2.1.2 Distribution and Known Occurrences/Survey Data ................................................................... 11
2.2 Species Ecology ................................................................................................................................. 17
2.2.1 Individual Level Ecology ............................................................................................................. 17
2.2.2 Population level ecology ............................................................................................................ 19
2.2.3 Synopsis of Species Ecological needs ......................................................................................... 22
3.0 Factors Influencing the Status of the Species ....................................................................................... 23
3.1 Stressors ............................................................................................................................................ 23
3.1.1 Pathogens .................................................................................................................................. 24
3.1.2 Pesticides ................................................................................................................................... 28
3.1.3. Habitat Loss and Degradation ................................................................................................... 32
3.1.4 Livestock Grazing ....................................................................................................................... 35
3.1.5 Climate Change .......................................................................................................................... 36
3.1.6 Small Population Dynamics ........................................................................................................ 37
3.1.7 Competition from non-native bees ............................................................................................ 38
3.2 Synergistic Effects ............................................................................................................................. 39
3.3 Beneficial Actions .............................................................................................................................. 39
4.0 Analysis of Current Condition ............................................................................................................... 40
5
5.0 Analysis of Future Condition ................................................................................................................. 42
6.0 Literature Cited ..................................................................................................................................... 43
7.0 Appendices ............................................................................................................................................ 63
FIGURES AND TABLES
FIGURE 1. ALL KNOWN OCCURRENCES OF B. FRANKLINI BY YEAR OF OBSERVATION (XERCES AND THORP 2010,
BROOKS 1999; CODE AND HANEY 2006, P. 3; POOL 2014, ENTIRE; COLYER 2016, ENTIRE; HATFIELD, PERS.
COMM. 2017; THORP, PERS. COMM. 2017) ....................................................................................................... 13
FIGURE 2. ALL KNOWN OCCURRENCES OF B. FRANKLINI, FROM 1923 TO THE PRESENT ........................................... 14
FIGURE 3. HISTORIC OBSERVATIONS AND SURVEY EFFORT……………………………………….……………………………………………16
TABLE 1. THE ECOLOGICAL REQUISITES FOR SURVIVAL AND REPRODUCTIVE SUCCESS OF B. FRANKLINI
INDIVIDUALS. ...................................................................................................................................................... 19
TABLE 2. THE REQUISITES FOR SURVIVAL AND REPRODUCTION SUCCESS OF B. FRANKLINI POPULATIONS. ............. 22
TABLE 3. ECOLOGICAL REQUIREMENTS FOR SPECIES-LEVEL VIABILITY IN B. FRANKLINI ............................................ 23
TABLE 4. ACRES OF AGRICULTURAL CROPLAND AND TOTAL ACRES ........................................................................... 33
TABLE 5. HUMAN POPULATION GROWTH ESTIMATES FOR DOUGLAS, JACKSON, AND JOSEPHINE COUNTIES IN
OREGON AND ASHLAND, OREGON. .................................................................................................................... 34
LIST OF ABBREVIATIONS
SSA Species Status Assessment
ESA Endangered Species Act
N Population Size
N
e
Effective Population Size
λ lambda, population growth rate
3Rs Resiliency, Representation, and Redundancy
spp. Species
USFS United States Forest Service
FWS Fish and Wildlife Service
BLM Bureau of Land Management
EPA United States Environmental Protection Agency
6
1.0 Introduction, Analytical Framework, and Methods
1.1 Introduction
This report presents the species status assessment (SSA) conducted for the Franklin’s bumble
bee (Bombus franklini). We, the Fish and Wildlife Service (Service), were petitioned to list B.
franklini as endangered under the Endangered Species Act of 1973, as amended (ESA), on June
23, 2010, by the Xerces Society for Invertebrate Conservation and Dr. Robbin Thorp, Professor
Emeritus from the University of California (Xerces Society and Thorp 2010, p. 2). In September
2011, the Service announced in the Federal Register that the petition presented substantial
information indicating that this species may be warranted for listing, and announced the
beginning of a status review for the species (Fish and Wildlife Service 2011). This SSA will be
the biological underpinning of the status review and the Service’s forthcoming 12-month finding
on whether B. franklini warrants protection under the ESA.
This SSA assesses the viability of Bombus franklini; that is, the likelihood that the species will
sustain populations over time. To assess B. franklini’s viability, we used the three conservation
biology principals of resiliency, representation, and redundancy (Shaffer and Stein 2000, pp.
308-311). These principals are described in general terms below, and more specifically for B.
franklini in section 4.0. Our approach for assessing B. franklini’s viability involved three stages.
In Stage 1, we describe the species ecology in terms of the 3Rs, identifying the ecological
requirements for survival and reproduction at the individual, population, and species levels. In
Stage 2 we use these ecological requirements to determine the baseline condition for the species
by assessing the species historical and current condition in relation to the 3Rs, and identifying
past and ongoing factors that led to the species current condition. Finally, in Stage 3 we use both
the baseline conditions as well as forecasts of the future levels of influence factors to project the
future condition of B. franklini.
Although there is abundant information available on the sub-genus Bombus sensu stricto, there is
very limited information available on B. franklini in particular. In 2016, the Service completed
the Rusty Patched Bumble Bee (Bombus affinis) Species Status Assessment (U.S. Fish and
Wildlife Service 2016a). Bombus franklini shares a close evolutionary relationship and shared
natural history traits with B. affinis (S. Colla, York University, Toronto, Ontario, Canada, 2018,
pers. comm.). We note that despite this taxonomic relationship there are distinct differences
between the species, particularly the more restricted range and limited distribution of B. franklini
compared to B. affinis. However, based on the close taxonomic relationship, B. affinis has been
identified as an acceptable proxy species to use in our assessment of B. franklini (R. Thorp,
University of California, Davis, California, pers. comm., 2017; Williams et al 2014, p. 114;
Goulson 2010, pp. 188-189; Thorp 2004; Schroeder pers. comm. 2017; Hatfield pers. comm.
2017). Due to the limited information on B. franklini, and in an effort to avoid duplicating effort
when assessing two very similar species, our SSA incorporates a portion of the information and
text provided in the rusty patched bumble bee SSA. Additionally, we note that the western
bumble bee (B. occidentalis) is also a member of the sub-genus Bombus sensu stricto, and shares
a portion of the range; therefore we also rely on information related to B. occidentalis for this
assessment of the status of B. franklini.
7
1.2 Analytical Framework
To assess the viability of Bombus franklini, we applied the conservation biology principles of
resiliency, representation, and redundancy (henceforth, 3Rs). Viability is the likelihood that the
species will sustain populations over time. To do this, a species must have a sufficient number
and distribution of healthy populations to withstand changes in its biological (e.g., novel
diseases, predators) and physical (e.g., climate change) environment, environmental stochasticity
(e.g., wet or dry, warm or cold years), and catastrophes (e.g., severe and prolonged droughts).
Viability is not a single state — viable or not viable; rather, there are degrees of viability--less to
more viable, or low to high viability. As the resiliency, representation, and redundancy of a
species increases, the species is better protected against the vagaries of the environment, and thus
it can better tolerate stressors (one or more factors that may be acting on the species or its
habitat, causing a negative effect). When the 3Rs increase, a species is more able to adapt to
future changes, and therefore, it is more viable. The 3Rs framework (assessing the health,
number, and distribution of B. franklini populations relative to frequency and magnitude of
environmental stochasticity and catastrophic events across its historical range of adaptive
diversity) is useful for describing the species’ degree of viability through time.
1.2.1 Resiliency
Resiliency is the ability of a species to sustain populations in the face of environmental variation
and transient perturbations. Environmental variation includes normal year-to-year variation in
rainfall and temperatures, as well as unseasonal weather events. Perturbations are stochastic
events such as fire, flooding, and storms. To be resilient, a species must have healthy populations
that are able to sustain themselves through good and bad years. Resiliency increases as the
number of individuals and populations increase, and the amount and distribution of available
habitat increases. For many species, resiliency is also affected by the degree of connectivity
among populations and the diversity of occupied ecological niches. Connectivity among
populations increases the genetic health of individuals (heterozygosity) within a population.
Furthermore, by increasing the potential for immigration, connectivity enhances a population’s
ability to recover from disturbances. Diversity of climate niches improves a species’ resiliency
by guarding against disturbances and perturbations affecting all populations similarly (i.e.,
decreases the chance of all populations experiencing bad years simultaneously or to the same
extent).
1.2.2 Representation
Species-level representation is the ability of a species to adapt to near and long-term changes in
the environment; it is the evolutionary capacity or flexibility of a species. Representation is the
range of variation found in a species, and this variation--called adaptive diversity--is the source
of species’ adaptive capabilities. Representation is therefore measured through the breadth of the
species’ adaptive diversity. The greater the adaptive diversity, the more responsiveness and
adaptability the species will have over time, thereby enhancing its viability. Maintaining adaptive
diversity includes conserving both the ecological and genetic diversity of a species. By
maintaining these two sources of adaptive diversity across a species’ range, the responsiveness
8
and adaptability of a species over time is preserved. Ecological diversity is the physiological,
ecological, and behavioral variation exhibited by a species across its range. Genetic diversity is
the number and frequency of unique alleles within and among populations.
In addition to preserving the breadth of adaptive diversity, maintaining evolutionary capacity
requires maintaining the evolutionary processes that drive evolution; namely, gene flow, genetic
drift, and natural selection. Gene flow is expressed through the physical transfer of genes or
alleles from one population to another through immigration and breeding. The presence or
absence of gene flow can directly affect the size of the gene pool available. Gene flow will
generally increase genetic variation within populations by bringing in new alleles from
elsewhere, but decrease genetic variation among populations by mixing their gene pools (Hendry
et al. 2011, p. 173). Genetic drift is the change in the frequency of alleles in a population due to
random, stochastic events. Genetic drift always occurs, but is more likely to negatively affect
populations that have a smaller effective population size (N
e
) and populations that are
geographically spread and isolated from one another. Natural selection is the process by which
heritable traits can become more (selected for) or less (not selected for) common in a population,
based on the reproductive success of an individual with those traits. Natural selection influences
the gene pool by determining which alleles are perpetuated in particular environments. This
selection process generates the unique alleles and allelic frequencies reflecting specific
ecological, physiological, and behavioral adaptations optimized for survival in different
environments.
1.2.3 Redundancy
Species-level redundancy is the ability of a species to withstand catastrophic events. Redundancy
protects species against the unpredictable and highly consequential events for which adaptation
is unlikely. In short, it is about spreading the risk. Redundancy is best achieved by having
multiple populations widely distributed across the species’ range. Having multiple populations
reduces the likelihood that all populations are affected simultaneously. The more widely
distributed populations are, the less likely they are to possess similar vulnerabilities to a
catastrophic event. Given sufficient redundancy, single or multiple catastrophic events are
unlikely to cause the extinction of a species. Thus, the greater redundancy a species has, the
more viable it will be. Furthermore, a greater number of populations and a greater diversity and
distribution of those populations, the more likely it is that the adaptive diversity and evolutionary
flexibility of the species will be preserved.
1.3 Methods
We gathered information to assess the viability of Bombus franklini from a variety of sources,
including the information in the 2010 Petition, our previous Federal Register notices, and our
files. In addition, we requested information from a diverse but specific audience, seeking
information on the species as well as all recent survey data from land managers and Federal
agencies. We also conducted a limited expert elicitation to collect more information and solicit
opinion on the species’ population dynamics. This elicitation included an extended interview
with Dr. Robbin Thorp, one of the petitioners and the noted species expert on B. franklini.
Additionally, we sent a questionnaire to 3 other professionals with experience and knowledge of

9
B. franklini (Richard Hatfield, Xerces Society; Peter Schroeder, Southern Oregon University;
and Pepper Trail, US Fish & Wildlife Service). A copy of the questionnaire can be found in
Appendix 4. We incorporated information from this elicitation effort into our analysis.
Building on the occurrence data provided in the 2010 Petition (Xerces Society and Thorp 2010,
Appendix 1) and other information gathered during our assessment, we assembled an occurrence
table and associated database of all known Bombus franklini occurrences, including information
provided by the Petitioners; information available in university and museum collections; and in
response to our requests (see Appendix 1). The table should not be considered a good
representation of actual numbers of B. franklini on the landscape because the data used to
assemble the occurrence table and database were generally collected through unsystematic,
opportunistic surveys and reporting, especially prior to 1998 (Thorp, University of California at
Davis, Davis, California, pers. comm. 2017), making it difficult to compare the number of
occurrences over time. The SSA for B. affinis generated a very rough estimate of the area of
habitat required to support a viable population of B. affinis by creating a post hoc systematic
sampling method (U.S. Fish and Wildlife Service 2016a, p. 11). This method entailed overlaying
a 10 km x 10 km grid across the range of the species and assigning a unique numerical identifier
and a textual description of the year(s) B. affinis were detected within that grid. We do not have
sufficient data on B. franklini occurrences over a similar spatial and temporal extent to conduct a
similarly comprehensive estimate. However, we do draw some conclusions about minimum
habitat requirements for B. franklini, as described in section 2.2.2.
Although we have evidence of the presence of Bombus franklini in certain areas, the lack of
systematic surveys across the historic range of the species over time prevents us from using these
occurrences to extrapolate reasonable estimates of species abundance or distribution. Many of
the occurrence records just provide point data for an occurrence, with no details on the size of the
area searched or whether or not the record reflected a comprehensive search of an area. Many
records also lack details on the level of survey effort per location (number of searchers, hours of
search effort per day, number of days per search effort). Additionally, because bumble bee nest
locations vary year-to-year, tracking individual colonies, and thus populations, over time is very
difficult. We cannot draw any conclusions on the abundance of B. franklini colonies or
population overall, since information is not available on how many individuals make up a
population (Thorp, pers. comm. 2017; P. Schroeder, pers. comm. 2017; R. Hatfield, Xerces
Society, Portland, Oregon, pers. comm. 2017). More targeted surveys were conducted in recent
years by those interested in the apparent decline of B. franklini, but they were not systematic and
only conducted in a limited number of specific sites throughout the species’ historic range. More
recent search efforts have primarily occurred on Federal land, however surveys have occurred
opportunistically on private land when access has been granted. Although it is possible that the
species may be extinct (University of California 2009), B. franklini colonies could potentially
persist in places that have not been systematically surveyed. A close relative, B. occidentalis,
was recorded in the Ashland, Oregon area in 2010, and not seen again in that area until two
individuals were observed during the focused surveys in July 2016 on Mt. Ashland (Thorp, pers.
comm. 2017).
10
2.0 Species Information
2.1 Background
2.1.1Taxonomy and Species Description
All of the approximately 250 species of bumble bees found worldwide (Williams et al. 2008, p.
1) belong to the genus Bombus (formerly Bremus), family Apidae, and order Hymenoptera, and
thirty species of Bombus are known in the western United States (Koch et al. 2012, entire).
Bombus franklini was first described in 1921, based on the collection of two queen specimens on
July 7, and July 8, 1917, in Nogales, Arizona (Frison 1921, pp. 147-148). The description of the
species was completed in 1922, based on one worker and one male specimen collected from an
unspecified locality in Oregon, and deposited in the United States National Museum (Frison
1923, p. 313-315; Thorp et al. 2010, pp. 5, 40). At that time, it was noted that B. franklini was
one of the rarer species of the widely distributed Bombus (Bremus) genus (Frison 1923, p. 315).
In 1970, based on museum record research and field studies, the actual location of the Nogales,
Arizona collection was called into question, and Gold Hill, Oregon, was proposed instead as the
type locality for Bombus franklini (Thorp 1970, p. 177-179; Thorp et al. 2010, p. 5, 7).
Several studies have been published on the taxonomic relationship of B. franklini to other
bumble bees (Stephen 1957, pp. 79-81; Milliron 1971, pp. 58-67; Plowright and Stephen 1980,
pp. 475–479; Thorp et al. 1983, pp. 29-30; Scholl et al. 1992, pp. 46-51; Cameron et al. 2007, p.
173). With the exception of Milliron (1971), who assigned B. franklini subspecific status under
B. terricola occidentalis, all of these studies have accorded B. franklini its own specific rank and
B. franklini is listed in the most recent world checklist of bumble bee species (Williams 1998, p.
129; Thorp et al. 2010, p. 5). Bombus franklini is also recognized as a valid species in the
Integrated Taxonomic Information System (Integrated Taxonomic Information System 2017).
For these reasons, we recognize B. franklini as a valid species and therefore, a potentially listable
entity under the ESA.
As a bumble bee of the subgenus Bombus sensu stricto, B. franklini is corbiculate (females
having pollen baskets on the hind legs) (Williams, et al. 2008, entire). In B. franklini, the hind
leg tibia outer surface (corbicula) is flat with long black fringes at the sides (Williams et al.
2014, p. 119). The species is short-tongued with a short head and the cheek (area between the
bottom of the compound eye to the insertion of the mandible) is shorter than it is wide (Koch et
al. 2012, p. 98; Williams et al. 2014, p. 119). Shorter faces and tongues are an adaptation to
extracting nectar from flowers with short corollas (Koch et al. 2012, p. 6). Bombus from this sub-
genus with short tongues also rob nectar from flowers with longer corollas, by biting holes in the
base of the corolla to access the nectar. Bombus occidentalis, a closely related species, has
mandibles with distinct teeth, possibly to aid in this behavior (Goulson 2010, p. 173). Body size
of the queens (22-24 mm, 0.86-0.95 inches) and workers (10-17 mm, 0.40-0.65 inches) is
relatively large (Williams et al. 2014, p. 119). Males are 13-16 mm (0.50-0.64 inches) in length.
In the field, B. franklini can most easily be distinguished from other similar species in its range
(e.g., B. occidentalis, B. vosnesenskii, B. caliginosus, B. vandykei, B. fervidus, B. insularis, B.
flavidus), by the inverted U-shape pattern of the yellow hairs on the anterior thorax surrounding a
11
central black patch and extending beyond the bases of the wings, and the lack of yellow hairs on
the abdomen (Thorp et al. 2010, p. 5-6; Williams et al. 2014, p. 119). In addition, the hairs on
the round face are predominantly black, there are yellow hairs on the top of the head, and there
are white hairs in two spots at the tip of the abdomen (Thorp et al. 2010, p. 5-6). For other
diagnostic characters that can be seen in the hand and under the microscope, please see Frison
(1921, pp. 147-148; 1922, pp. 313-315), Thorp et al. (2010, pp. 5-6), and Williams et al. (2014,
pp. 119-120).
2.1.2 Distribution and Known Occurrences/Survey Data
Bombus franklini is thought to have the most limited distribution of all known North American
bumble bee species (Plowright and Stephen 1980, p. 479; Xerces Society and Thorp, 2010, p. 6),
and one of the most limited geographic distributions of any bumble bee in the world (Frison
1923, p. 315; Williams 1998, p.129). Stephen (1957, p. 81) recorded the species from the
Umpqua and Rogue River Valleys in Oregon. Thorp et al. (1983, p. 8) also recorded it from
northern California and suggested its restriction to the Klamath Mountain region of southern
Oregon and northern California. Elevations where it has been observed range from 162 m (540
feet) in the northern part of its range, to over 2,340 m (7,800 feet) in the south of its historical
range. All confirmed specimens have been found in an area about 306 km (190 miles) to the
north and south, and 70 miles 113 km (70 miles) east to west, between 122° to 124° west
longitude and 40° 58’ to 43° 30’ north latitude in Douglas, Jackson, and Josephine counties in
southern Oregon, and Siskiyou and Trinity counties in northern California (Thorp 1999, p. 3;
Thorp 2005c, p. 1; International Union for Conservation of Nature 2009, p. 1). Twenty three of
the 43 sites where B. franklini has been located are privately owned, 18 are on Federal land (U.S.
Forest Service and Bureau of Land Management), one site is on State land, and one is on
municipal land.
Limited occurrence and observation data exist for Bombus franklini prior to 1997. Historic
observations and occurrence data includes randomly reported observations, student collections,
and museum specimens, as well as the collections and notes of interested parties, natural
resource managers, and university staff (Xerces Society and Thorp 2010, pp. 34-40). As
mentioned in the previous section, B. franklini was first observed in 1917 and first described in
1921. Between 1923 and 1992 there were 31 additional occurrences recorded in Oregon, and
seven recorded in California. Of the 38 records, 25 noted five or less bees, and only one 1968
record counted more than 12 bees at a single location (Appendix 1). For many of the occurrences
between 1923 and 1992, we do not have an understanding of whether or not the
surveyors/collectors were noting all of the B. franklini observed at that site on that day. No
survey methodologies were reported so we do not know how surveyors/collectors looked for the
bees at the various sites or how long they spent looking at a given site on a given day.
Furthermore, information about search efforts that took place where no bees were detected
(negative occurrence data) would not be on record (Thorp, pers. comm. 2017). Therefore, the
main information that B. franklini records from this period provide is documentation of presence
of the species at a given location; they do not provide a clear understanding of historic
population abundance across the range. However, Dr. Robbin Thorp has noted that in the 1960’s,
when he looked at sites where he thought B. franklini might be, he was able to find the species.
12
He also suspects that if others knew where to look for the species, they would have been likely to
find the species at the time (Thorp, pers. comm. 2017).
In 1997, there were two records of Bombus franklini in Oregon, each noting two bees counted.
Also that year, three queen B. franklini and nine workers were observed in the Marble Mountains
(Siskiyou County, California) study area, by a master’s student from Humboldt State University
(M. Brooks, Humboldt State University, Arcata, California, pers. comm. 1997). The study did
not survey for B. franklini specifically, but was looking at Bombus assemblages and flower
preferences based on tongue-length. Bombus franklini was observed at six specific locations
(three locations each, on two of the study’s ten total survey areas), between June 6
th
and August
15
th
; the bees were observed on lupine (Lupinus spp.), mountain monardella (Monardella
odoratissima), and clover (Trifolium spp.) (Brooks pers. comm. 1997; Brooks 1999, p. 11).
A survey effort specifically focused on Bombus franklini began in 1998 and continues annually,
at sites representing both a subset of historical and potential new localities for the species.
According to the information provided in the 2010 petition (Xerces Society and Thorp, 2010, p.
7), between nine and 17 historical sites (averaging 13.8 sites annually), and two to 23 additional
sites, were surveyed each year from 1998 until 2010. Some sites were visited more than once per
year, or in multiple years, and some historic locations have not been resurveyed since the
original observation of B. franklini at that location. These surveys were primarily focused on
localities in Jackson County, Oregon around the center of the historic range (Xerces Society and
Thorp, 2010, p. 9; Thorp, pers. comm. 2017). During the surveys from 1998 to 2006, B. franklini
was observed at 11 sites, including seven sites where it had not been previously documented. In
1998, 98 individuals were located at eight sites (with 81 of those individuals occurring at two of
the eight sites). In 1999, only 20 individual bees were located. Nine individuals were observed in
2000, and one individual in 2001. Although 20 were observed in 2002, only three were observed
in 2003 (all at a single locality), and a single worker bee was observed in 2006. There have been
no confirmed observations of B. franklini since the single worker in 2006. Figure 1 displays a
graph of all known presence data for B. franklini over time, from the first observation in 1923
until 2017 (2006 was the last documented occurrence). Figure 2 displays a map of all known
occurrence data for B. franklini, from 1923 to the present.

13
Figure 1. All known occurrences of Bombus franklini by year of observation (Xerces and Thorp 2010, Brooks 1999; Code and Haney
2006, p. 3; Pool 2014, entire; Colyer 2016, entire; Hatfield, pers. comm. 2017; Thorp, pers. comm. 2017)
YEAR
0
10
20
30
40
50
60
70
80
90
100
1923
1925
1927
1929
1931
1933
1935
1937
1939
1941
1943
1945
1947
1949
1951
1953
1955
1957
1959
1961
1963
1965
1967
1969
1971
1973
1975
1977
1979
1981
1983
1985
1987
1989
1991
1993
1995
1997
1999
2001
2003
2005
2007
2009
2011
2013
2015
2017
Bombus franklini records from 1923 to 2017
More intensive survey
effort began (1998)
Number of bees reported

14
Figure 2. All known occurrences of Bombus franklini, from 1923 to 2017.
15
In 2006, the Bureau of Land Management conducted a survey of 16 sites on the Mt. Ashland
resource area in the Medford District that were believed to provide optimal habitat for Bombus
franklini. Each site was surveyed twice by trained technicians, but no B. franklini were found
(Code and Haney 2006, p. 3).
Since 2009, a number of targeted surveys have taken place at select locations within the historic
range of Bombus franklini, in an effort to locate the species and other rare or declining
invertebrates (including Western bumble bee (occidentalis)). In 2014, the Medford District of
BLM conducted a survey for six special status meadow invertebrates, including B. franklini and
B. occidentalis. Surveys were conducted between July and September, with survey locations
based on (1) historical occurrence records for private, BLM and USFS lands, and (2) water and
floral resources. Bombus occidentalis was observed at three locations; no B. franklini were found
(Pool 2014, entire). The surveys were conducted in areas that appeared to have good quality
habitat for Bombus (S. Godwin, Bureau of Land Management, Medford, Oregon, pers. comm.,
2017).
Surveys targeting Bombus occidentalis took place on the Umpqua and Rogue River-Siskiyou
National Forests in 2015 and 2016, with trained observers covering dozens of historical locations
with a wide variety of habitat types and elevations throughout the flight season. Over a dozen
Bombus species were recorded including B. occidentalis, but no observations of B. franklini were
made (Colyer 2016, entire). Generally the surveys were conducted in habitat that would be good
for B. franklini, given that all of the sites had several different Bombus spp. detections (S.
Colyer, U.S. Forest Service, Prospect, Oregon, pers. comm. 2017).
In response to our request for information, the Xerces Society provided records of all Bombus
observations reported to Bumblebeewatch.org, between 2012 and 2017, within the historical
range of B. franklini. All reports are from incidental observations and have been confirmed by
taxonomic experts. Over 100 observations of Bombus spp. were reported from a wide variety of
land ownerships, habitats and elevations, and included 18 different Bombus species (including B.
occidentalis), however no B. franklini were observed (Hatfield, pers. comm. 2017). While this
information is not part of a standardized survey, it does represent some level of opportunistic
observation and reporting opportunity available over time within the historical range of the
species, and all are observations were verified by experts; thus we feel it is worth mentioning.
Again, no new observations of B. franklini have been made since 2006.
As mentioned earlier, Bombus occidentalis was recorded in the Ashland, Oregon area in 2010,
and not seen again in that area during the annual surveys until two individuals were observed
during the focused surveys in July 2016 on Mt. Ashland (Thorp, pers. comm. 2017). Bombus
occidentalis, like B. franklini, is not migratory and therefore must have been present in the
survey area, yet remained undetected during the surveys over multiple sequential years. This is
indicative of the low detection probability for these rare species, even when focused annual
survey efforts by trained observers are taking place.
Figure 3 below displays all the sites on record that have been surveyed for Bombus franklini.
This includes survey location information from the Bureau of Land Management, U.S. Fish and

16
Wildlife Service, and the U.S. Forest Service; information from the 2010 Petition (Xerces
Society and Thorp 2010, p. 34-40; additional information from Dr. Robbin Thorp (Thorp, pers.
comm. 2017) and Brooks (Brooks 1997, p. 4), as well as all Bombus observations within the
historic range of the species from 2012-2017 as reported in Bumblebeewatch.org (Hatfield pers.
comm. 2017).
Figure 3. All sites surveyed for Bombus franklini from 1923 to 2017.
17
2.2 Species Ecology
2.2.1 Individual Level Ecology
The specific life history characteristics or behavior of this rare species have not been studied. As
one of the rarest Bombus species, B. franklini are somewhat enigmatic and a specific habitat
study for the species has not been completed. Such a study was initiated in 2006 when B.
franklini was last seen, but could not continue due to the subsequent absence of the species
(Thorp 2017, pers. comm.). While little is known about B. franklini’s reproductive biology,
specific habitat needs or unique behavior, this information is available for Bombus in general and
for some closely-related species (B. occidentalis, B. affinis, and B. vosnesenskii, among others).
Bombus franklini is a primitively eusocial bumble bee, living in colonies made up of a queen and
her offspring – males and workers. The nesting biology of B. franklini is unknown (Xerces
Society and Thorp 2010, p. 10), but they likely nest underground in abandoned rodent burrows or
similar cavities that offer resting and sheltering places, food storage, nesting and room for the
colony to grow, as is typical for other eusocial Bombus species (Plath 1927, pp. 122-128; Hobbs
1968, p. 157; Thorp et al. 1983, p. 1; Thorp 1999, p. 5). It may also occasionally nest on the
ground (Thorp et al. 1983, p.1) or in rock piles (Plowright and Stephen 1980, p. 475), and has
even been found nesting in a residential garage in the city limits of Medford, Oregon (Thorp
2017, pers. comm,).
The flight season of Bombus franklini is from mid-May to the end of September (Thorp et al
1983, p. 30); a few individuals have been encountered in October (Southern Oregon University
Bee Collection records, in Xerces Society and Thorp, 2010, Appendix 1 page 39). Colonies of B.
franklini have an annual cycle, initiated each spring when solitary queens emerge from
hibernation and seek suitable nest sites (Thorp, pers. comm. 2017). The queen collects nectar and
pollen to support the production of her eggs, which are fertilized by sperm she has stored
throughout hibernation since mating the previous fall. In the early stages of colony development,
the founding queen (foundress) is responsible for all food collection and care of the eggs and
larvae. As the colony grows, workers assume the duties of food collection, colony defense, nest
construction, and larval care while the foundress remains within the nest and produces eggs.
Colonies of B. franklini may contain from 50 to 400 workers, and the founding queen (Plath
1927, pp. 123-124; Thorp et al 1983, p. 2; Macfarlane et al 1994, p. 7). Two colonies of B.
franklini initiated in the laboratory and set out to complete development in the field contained
over 60 workers by early September, and likely produced over 100 workers by the end of the
season (Plowright and Stephen 1980, p. 477).
Near the end of the colony cycle, reproductive queens (gynes) and fertile males are produced.
Male bumble bees patrol selected territories, which they mark with queen-attracting scent.
Queens locate a territory and remain still until a male finds her. Mating usually takes place on the
vegetation on or near the ground. Queens usually mate with only one male, but males may mate
with multiple females who enter the territory. After mating, queens feed to build up fat before
entering hibernation. At the end of the colony cycle, all the workers and the males die along with

18
the founding queen; only the inseminated hibernating gynes are left to carry on the line into the
following year (Duchateau and Velthius 1988). Over wintering habitat would include micro-
habitats such as ground cavities, rotting logs, loose soil and other protected sites for queens to
hibernate, with floral resources and suitable nest sites available for the emerging queens the
following spring. Mating habitat requirements for most bumble bee species is not known.
Bumble bees are generalist foragers, meaning they gather pollen and nectar from a wide variety
of flowering plants (Xerces Society 2013, pp. 27-28). Bumble bees are very efficient at
collecting pollen; unlike honey bees, they often vibrate their flight muscles while inside a flower,
causing pollen to fall from the plant anthers and stick to the bumble bee’s copious body hairs.
This behavior of “buzzing” a flower is also known as sonication, and is one of the characteristics
of bumble bees that make them particularly attractive for commercial pollination; bumble bees
can pollinate flowers hundreds of times faster than honey bees (Williams et. al. 2014, p. 16).
Bombus franklini requires a constant and diverse supply of flowers that bloom throughout the
colony’s life cycle, from spring to autumn (Xerces Society and Thorp 2010, p. 11); these
resources would typically be found in open (non-forested) meadows in proximity to seeps and
other wet meadow environments. Different Bombus species have consistently been observed
foraging in the same area visiting similar and different species of flowering plants. During some
Oregon surveys, no Bombus species was always consistent in the number of different plants
species it visited, nor was any Bombus species tied to just one plant species (Schroeder, pers.
comm. 2017). The nectar from flowers provides carbohydrates and the pollen provides protein.
Studies of other Bombus species typically exhibit foraging distances of less than 1 km (0.62
miles) from their nesting sites (Knight et al. 2005, p. 1816; Wolf and Moritz 2008, p. 422;
Dramstad 1996, pp. 163-182; Osborne et al. 1999, pp. 524-526; Rao and Strange 2012, pp. 909-
911; Hatfield, pers. comm. 2017). Bombus franklini may have a foraging distance of up to 10 km
(6.2 miles) (Thorp, pers. comm. 2017), but the subgenus’ typical dispersal distance is most likely
3 km (1.86 miles) or less (Hatfield, pers. comm. 2017; Goulson 2010, p. 94,). Bombus franklini
have been observed collecting pollen from lupine (Lupinus spp.) and California poppy
(Eschscholzia californica), and collecting nectar from horsemint or nettle-leaf giant hyssop
(Agastache urticifolia) and mountain monardella (Monardella odoratissima) (Xerces Society and
Thorp 2010, p. 11). Bombus franklini may also collect both pollen and nectar from vetch (Vicia
ssp.) as well as rob nectar from it (Xerces Society and Thorp 2010, p. 11). A short-
tongued/cheeked bumble bee, B. franklini has been found to antagonistically rob nectar from
flowering plants that it cannot directly reach with its tongue, by chewing a hole in the host plant
where the nectar is located (Pool 2014, p. 3; Schroeder, pers. comm. 2017; Hatfield, pers. comm.
2017). This particular behavior has been known to occur during its visitation to pollinator plants
such as Aconitum. Table 1 summarizes ecological requirements of B. franklini at the individual
level.

19
Table 1. The ecological requisites for survival and reproductive success of Bombus franklini
individuals.
Life Stage
Winter
Spring
Summer
Autumn
Queen
Diverse floral
resources;
suitable
nest habitat
Diverse floral
resources;
suitable
nest habitat
Diverse floral
resources; suitable
nest habitat
Worker Females
Diverse floral
resources in
close
proximity to
nest
Diverse floral
resources in
close
proximity to
nest
Diverse floral
resources in close
proximity to nest
Males
Diverse floral
Resources;
suitable mating
habitat
Diverse floral
resources; suitable
dispersal/mating
habitat
Gynes (new
foundress queens)
Suitable
diapause sites
Diverse floral
resources
Diverse floral
resources; suitable
dispersal/mating
habitat
In summary, Bombus franklini has been found in a wide array of sheltered and exposed habitat
types at a broad elevational range, and the species appears to be a generalist forager. Our
certainty regarding B. franklini habitat needs is limited to (1) floral resources for nectaring
throughout the colony cycle, and (2) relatively protected areas for breeding and shelter. The
habitat elements that B. franklini appears to prefer to fulfil those needs mentioned above are
relatively flexible, plentiful, and widely distributed.
2.2.2 Population level ecology
Bombus franklini has long been considered a rare or vary rare species, with a relatively small
population size and relatively small colony size compared to other Bombus species (Thorp, pers.
comm. 2017; Hatfield, pers. comm. 2017). No more than 356 individuals have been observed in
total, and no more than 98 total individuals at eight separate locations have been observed in any
one year (Xerces Soc. and Thorp 2010, p. 7; Occurrence Table, Appendix 1). We have no
definitive information on the minimum number of colonies or minimum habitat patch size for a
self-sustaining population of B. franklini. As stated above in section 1.3 the assessment for B.
affinis created a 10 kilometer (km) x 10 km (6.2 miles x 6.2 miles) grid across the range of the
species to generate a rough estimate of the area of habitat required to support a viable population
of B. affinis. The lack of information on B. franklini makes it unreasonable to do the same
comprehensive exercise for this species, however we can look at some general principles of B.
franklini life history to provide us with a very rough estimate of minimum habitat requirements
for our best guess of what constitutes a population of the species. If we focus on the minimum
area of habitat required to allow for individuals from different B. franklini colonies to travel their
typical foraging distance to forage at a common location and potentially interbreed, we find that

20
an area 6 km x 6 km might accomplish that. This spatial estimate is appropriate for B. franklini
for the following reasons: (1) the subgenus’ typical dispersal distance for B. franklini is 3 km
(Hatfield, pers. comm. 2017; Goulson 2010); (2) B. franklini individuals concurrently visiting a
site are often from different colonies (Hatfield, pers. comm. 2017); and, (3) colonies would have
to be within dispersal distance of other colonies in order to interbreed and maintain genetic
diversity. An area 6 km x 6 km (3.72 miles x 3.72 miles) would allow for the possibility that B.
franklini from different colonies that are 6 km (3.72 miles) apart could each disperse 3 km (1.86
miles) to a shared foraging location. It is, therefore, reasonable to assume that multiple B.
franklini detections over time within a 6 km x 6 km (3.72 miles x 3.72 miles) area would likely
represent a single population. This measure of 6 km
2
(3.72 square miles) is therefore a
reasonable estimate of minimum patch size for a self-sustaining population of B. franklini.
Population viability requires healthy demographics and sufficient habitat to support a healthy
demography; specifically, viability is a function of population size (N) and its population growth
rate (lambda, λ). The population structure of Bombus franklini operates similarly to a
metapopulation. A metapopulation is an assemblage of interacting subpopulations; a population
of B. franklini is a collection of interacting colonies. But, whereas a subpopulation is composed
of many reproductive individuals, a B. franklini colony is founded by a single queen, and thus a
colony represents one reproductive unit. The effective population size (N
e
) of B. franklini is,
therefore, the number of successful nests or colonies – not the number of individuals.
Population size also affects population viability through genetic health. Small populations have
lower levels of genetic diversity (heterozygosity), which reduces the capacity of a population to
respond to environmental change. Inbreeding depression may result, leading to reduced
longevity and fecundity and overall population fitness (Darvill et al. 2006, p. 602). Populations
of monoandrous social species like Bombus franklini (colonies headed by a single queen who
mates with a single male), are especially vulnerable to inbreeding depression, because the rate of
genetic drift in a population is determined by the effective population size (N
e
) which is much
lower than the number of individuals in an area (Goulson and Darvill 2008, pp. 197-198; Darvill
et al. 2006, p. 602). The N
e
in bumble bees is 1.5 times the number of successful nests, not 2
times, as is the case with diploid-diploid organisms (Goulson and Darvill 2008, pp. 197-198).
The reproductive system of bumble bees renders them particularly sensitive to loss of genetic
diversity. Bombus species exhibit haplodiploidy (i.e., males are haploid and females are diploid)
and exhibited a single locus complementary sex determination (sI-CSD) system (Zayed 2009, p.
238). Typically, heterozygotes at the sex-determining locus develop into diploid females from
fertilized eggs, while hemizygotes (a diploid individual with only one allele for a particular gene)
develop into haploid males from unfertilized eggs (Zayed 2009, p. 239). In cases, however,
where females mate with haploid males that share a sex-determining allele in common (called
“matched mating”), half of the females’ progeny will be homozygous at the sex-determining
locus and will consequently develop into diploid males instead of females. As males do not
contribute resources to the colony, homozygosity at the sex-determining locus imposes a cost to
the colony by decreasing the number of females produced (Ellis et al. 2006, p. 4376).
Additionally, diploid males are unviable, or if viable and mate, produce diploid sperm, which
will lead to unviable fertilized eggs or sterile triploid daughters (Zayed 2009, p. 239), so those
males that are produced are unable to contribute to next year’s cohort. Matched mating occurs
21
most often when allele diversity at the sex-determining locus is low (Ellis et al. 2006, p. 4376;
Zayed 2009, pp. 239-241). Thus, as N
e
decreases, the likelihood of producing diploid males
increase, which will further reduce the population size, potentially resulting in a negative,
reinforcing downward cycle (i.e., extinction vortex). Zayed and Packer (2005, pp. 10743-10744)
found, through modeling simulations, that extinction risks in haplodiploid populations were an
order of magnitude higher than probabilities of extinction due to inbreeding depression in diploid
populations. They attributed this high extinction risk to the effects of the “diploid male vortex”; a
phenomenon where diploid males initiate a positive feedback cycle that leads to rapid extinction.
Several species of bumble bee in England have demonstrated a dynamic consistent with this
negative, reinforcing pattern. Bombus subterraneous, for example, following reduction in
population size due to habitat loss eventually went extinct in the United Kingdom despite
continued suitability of habitat (Darvill et al. 2006, p. 608). Maintaining genetic diversity within
populations, thus, requires large N
e
and gene flow within and among populations.
The viability of a population is also determined by its long-term lambda; in order for any
population to persist over time, its growth rate, λ, must exceed 1.0. Species that fluctuate greatly
with environmental conditions, require strong lambdas over time to avoid extirpation. The
minimum λ needed to sustain a Bombus franklini population over time is unknown, but insects
are particularly susceptible to environmental stochasticity. Although bumble bees, because of
their relatively larger body size and fuzzy bodies, are not as strongly influenced by
environmental conditions as other insects including honey bees, climatic conditions affect the
availability of requisite resources, and hence, bumble bee numbers. Pollen and nectar
availability, especially in spring and fall when floral resources are scarcer, are influenced by
environmental conditions (Holm 1966, pp. 156-157); in years with unfavorable weather, the
supply of food is limited, leading to smaller and fewer colonies. Thus, population viability
requires occupying areas with a diversity of environmental conditions (spatial heterogeneity) to
ensure floral resources are available throughout the season and year-to-year despite variations in
climatic variables, such as temperature and precipitation. Similarly, spatial heterogeneity
increases the likelihood of asynchrony among colonies, a pre-requisite for metapopulation long-
term persistence (Hanski 1999, p. 28). In spatially heterogeneous populations, it is unlikely that
the entire population will contemporaneously experience the same environmental conditions,
thus ensuring that not all colonies comprising a population will fail due to unfavorable
conditions.
In summary, the significant determinants of population-level viability for Bombus franklini are a
healthy demography and sufficient quality habitat to support this demography. The demography
of B. franklini populations is a function of its population size (the number of successful nests)
and its population growth rate over time. The population size required to support a viable
population is likely variable across spatial scales and is unknown, but generally speaking, the
larger the population, the more genetically healthy and thus the more robust to extirpation.
Similarly, the minimum long-term λ required to sustain a population over time is unknown, but it
must exceed 1.0 and likely must be higher, given the susceptibility to environmental
stochasticity. Both of these variables, N and λ, are dependent upon the amount and quality of
floral resources, nest sites, and overwinter sites across temporal scales (within and among years).
A precise estimate of the area of habitat required to support a viable population is dependent on
the density and quality of floral resources, but given the large amount of food needed to support

22
successful colonies, it is reasonable to assume a large area is required. Another important aspect
of population viability is connectivity among colonies to ensure mating of unrelated reproductive
individuals and connectivity among populations to maintain within-population genetic diversity.
Lastly, the degree of spatial heterogeneity across the population area reduces the chances of all
colonies failing concurrently due to poor environmental conditions, and thus, is important for
long-term persistence. For B. franklini, we can estimate that a minimum area of 6 km
2
would
allow for individuals from different colonies to travel their maximum foraging distance to forage
at a common location and have the opportunity to interbreed. Based on the above, the ecological
requirements for successful population of B. franklini are listed below in Table 2.
Table 2. The requisites for survival and reproduction success of Bombus franklini populations.
Population Health (fitness)
Element
Importance
Healthy Demography
Large N
e
Multiple, successful colonies
Patch size at least 6 km
2
Successful colonies,
connectivity
Habitat connectivity
To find unrelated mates
Habitat to support healthy
demography
Sufficient floral resources
Adequate quantity of nectar
and pollen
Nesting and overwintering
sites
Safe breeding and shelter
Habitat connectivity
To safely and efficiently find
food
Heterogeneity
Diverse environmental
conditions
2.2.3 Synopsis of Species Ecological needs
Viability is the likelihood that a species will sustain populations over time. To do this, Bombus
franklini needs a sufficient number and distribution of self-sustaining populations to withstand
environmental stochasticity (resiliency), adapt to changes in its environment (representation),
and withstand catastrophes (redundancy) (Table 3).

23
Table 3. Ecological requirements for species-level viability in Bombus franklini
3Rs
Requisites of long-term
viability
Description
Resiliency
(able to
withstand
stochastic
events)
Interconnected, healthy
populations
across a diversity of
habitats
Populations with:
1) large N
e
, sufficient floral resources in close
proximity to nesting and overwintering sites,
2) connectivity among colonies, and
3) spatial heterogeneity; high connectivity
among populations dispersed across diverse
climatic conditions (spatial heterogeneity)
Representation
(to maintain
evolutionary
capacity)
Maintain adaptive
diversity of the
species
Healthy populations distributed across areas of
unique adaptive
diversity
Maintain evolutionary
processes
Maintain evolutionary drivers--gene flow,
natural selection, genetic drift- to mimic
historical patterns
Redundancy
(to withstand
catastrophic
events
Sufficient distribution of
healthy
populations
Sufficient distribution to guard against
catastrophic events wiping
out portions of the species adaptive diversity,
i.e., to reduce
covariance among populations
Sufficient number of
healthy populations
Adequate number of healthy populations to
buffer against
catastrophic losses of adaptive diversity
3.0 Factors Influencing the Status of the Species
Factors can influence a species both negatively and positively, as well as in synergy with other
factors. We focused our analysis on six primary stressors potentially negatively affecting the
species – pathogens, pesticides, habitat loss and degradation, grazing, climate change, and small
population dynamics. We then looked at potential synergistic effects between these stressors.
Finally, we looked at beneficial actions that may be positively affecting the condition of the
species.
3.1 Stressors
The 2010 Petition identified the following factors as stressors on Bombus franklini and its
habitat: introduced exotic diseases and competition from non-native bees; destruction,
degradation and conversion of habitat; pesticides and pollution; inadequacy of current rules,
regulations and law; introduction of exotic plant species; increased human use of native habitat;
climate change; and alteration of wildfire severity and frequency (Xerces Society and Thorp
2010, p. 4). In our 90-day finding on the 2010 Petition (U.S. Fish and Wildlife Service, 2011),
we noted that the petitioners provided substantial information on stressors to B. franklini from
the destruction, modification or curtailment of habitat (primarily due to the potential impacts of
natural or prescribed fire), disease, as well as the inadequacy of existing regulatory mechanisms
24
and other natural or manmade factors (including pesticides, small population dynamics,
competition from non-native bees, and climate change).
In this SSA, we analyzed the factors noted as leading to our substantial 90 day finding. In
addition, based on new information received, we looked again at agricultural intensification,
urban development, and livestock grazing, as well as synergistic effects of the stressors in
combination with each other. We discuss existing regulatory mechanisms and conservation
actions in section 3.3, Beneficial Actions.
3.1.1 Pathogens
A number of diseases are known to naturally occur in bumble bee populations. These include the
protozoan parasite Crithidia bombi, the tracheal mite Locustacarus buchneri, and the
microsporidium (parasitic fungus) Nosema bombi, as well as deformed wing virus. Pathogens
and parasites are widespread generalists in the host genus, but affect species differently
according to host susceptibility and tolerance to infection (Kissinger et al. 2011, p. 221; Malfi
and Roulston 2014, p. 18). The host species’ life history plays a role in the virulence of a given
pathogen; for instance, parasites may have relatively smaller effects on species with shorter
colony life cycles and smaller colony sizes (Rutrecht and Brown, 2009, entire).
Pathogen spillover is a process whereby parasites and pathogens spread from commercial bee
colonies to native bee populations (Colla et al. 2006, p. 461; Otterstatter and Thompson 2008, p.
1). The precipitous decline of certain Bombus species from the mid-1990s to present –
particularly species in the subgenus Bombus sensu stricto (including B. franklini) – was
contemporaneous with the collapse of commercially bred B. occidentalis, which were raised
primarily to pollinate greenhouse tomato and sweet pepper crops beginning in the late 1980s
(Szabo et al. 2012, pp. 232 -233). This collapse was attributed to N. bombi. Around the same
time, several North American wild bumble bee species – B. affinis, B. occidentalis, B. terricola
(all in the same subgenus Bombus sensu stricto), and B. pensulvanicus, also began to decline
rapidly (Szabo et al. 2012, p. 232).
Bumble bees are very efficient pollinators of a wide variety of crops, including fruits, nuts, and
vegetables (Loken 1958; Holm 1966b, Corbet et al. 1991, Cane and Payne 1993, MacKenzie and
Averill 1995; Goodell and Thomson 1997, Macfarlane and Patten 1997, Mayer and Lunden
1997, Stubbs and Drummond 2001, Thorp 2003). As mentioned in section 2.2.1, bumble bees
sonicate or “buzz pollinate” flowers hundreds of times faster than honey bees can. This attribute,
combined with their tolerance of temperature extremes and longer foraging seasons, make them
ideal for commercial greenhouse crop production (North American Pollinator Protection
Campaign 2006, p. 6). Roughly 95 percent of all commercially-reared bumble bee colonies are
used in the greenhouse production of tomatoes and sweet peppers (Velthuis and van Doorn 2006,
Shipp et al. 1994, Ercan and Onus 2003). Commercial bumble bee production started in North
America in the early 1990s (Xerces Society and Thorp 2010, p. 15). Queens of both Bombus
occidentalis and B. impatiens were shipped from the United States to rearing facilities in
Belgium that were also likely rearing Bombus terrestris (a closely related Bombus species native
to Europe). Bombus terrestris was also likely imported to Mexico in 1995 and 1996 for
greenhouse tomato pollination (Winter et. al. 2006, p.5).
25
The commercially-reared colonies produced from these queens were shipped back to the United
States between 1992 and 1994. Bumble bee producers experienced major problems with Nosema
bombi infection in commercial Bombus occidentalis in 1997 (Flanders et al. 2003, p. 108;
Velthius and van Doorn 2006, p. 432), and eventually stopped producing B. occidentalis. In
addition, the morphology of N. bombi found in a native bumble bee in China, Bombus leucorum,
was found to be the same as that found in B. terrestris imported into China from New Zealand
(Jilian et al. 2005, p. 53), suggesting the disease may have been introduced into native bumble
bee populations in China by commercial bees. Studies suggest that disease can be spread from
commercial bumble bees to nearby wild bumble bees (Niwa et al. 2004, p. 60; Whittington et al.
2004, p. 599; Jilian et al. 2005, p. 53; Colla et al. 2006, p. 461), even when commercial bumble
bees are used for pollination in greenhouses. This is because commercial bumble bees frequently
forage outside greenhouse facilities and can transmit disease at shared flowers (Xerces Society
and Thorp, 2010, p. 15; Whittington et al. 2004, p. 599; Colla et al. 2006, p. 461). In addition to
commercial pollination, B. occidentalis colonies were used in field research between 1991 and
2000 in California, Washington, and Alberta, Canada (Mayer et al. 1994, p. 21; Mayer and
Lunden 1997, p. 283; Richards and Myers 1997, p. 293; Mayer and Lunden 2001, p. 277;
Thompson 2004, p. 460).
Nosema bombi is a microsporidium (parasitic fungus) that has been detected in native bumble
bees in North America, and has been found to be a part of the natural pathogen load, reported in
Canada since the 1940s (Cordes et al. 2011, p.7) and appears to have a broad host range in North
America (Kissinger et al. 2011, p. 222). Nosema bombi infections primarily occur in the
malpighian tubules (small excretory or water regulating glands), but also in fat bodies, nerve
cells, and sometime the trachea (Macfarlane et al. 1995). Colonies can appear to be healthy but
still carry N. bombi and transmit it to other colonies. Transmission of N. bombi most likely
occurs when spores are fed to larvae (Eijnde and Vette 1993 and Rutrecht 2007, as cited in
Meeus et al. 2011, p. 666). Murray et al. (2013, p. 274 citing Rutrecht et al. 2007) notes that N.
bombi spreads slowly through novel populations with subsequent inter-colony infections through
drift of infected adults into non-natal colonies.
The effect of Nosema bombi on bumble bees varies from mild to severe (Macfarlane et al. 1995;
Rutrecht et al 2007, p. 1719; Otti and Schmid-Hempel 2008, p. 577). N. bombi can have large
effects on individual bees. Infected animals may have crippled wings, and queens may have
distended abdomens and be unable to mate (Otti and Schmid-Hempel 2007, pp. 122-123). Malfi
and Roulston (2014, p. 24) found that N. bombi infections are more frequent and more severe in
rare species and also that the species with the highest percentages of infected individuals were
rare species.
The Petitioners hypothesize that a virulent strain of Nosema bombi from Bombus terrestris
spread to B. impatiens and B. occidentalis prior to their shipment back into the United States, and
once in this country the commercially reared colonies may spread the virulent strain to wild
populations of B. franklini. In work partially funded by the U.S. Fish & Wildlife Service, surveys
for parasites and pathogens in bumble bee populations of the Pacific Northwest and Midwest
were conducted by the University of Illinois between 2005 and 2009. The goal was to assess
Bombus populations for presence and prevalence of pathogens, particularly microsporidia, in an
26
effort to provide baseline data to assess disease as a potential factor in the decline of B. franklini,
B. occidentalis, and B. pensylvanicus (Solter et al, 2010, p. 1). The highest prevalence of N.
bombi was found in B. occidentalis, with 26 percent of collected individuals infected. Crithidia
bombi infections of B. occidentalis were 2.8 percent overall. No B. franklini were collected
during the study. However, Mt. Ashland, Oregon (the last known location for B. franklini), was
one of only three sites in the Pacific Northwest study area where N. bombi infections were found
in multiple Bombus species (B. insularis and B. bifarius); the recovery of N. bombi infections
from multiple Bombus species at a site was otherwise rare (Solter et al 2010, pp. 3-4). Although
Cordes et al (2011, p. 7) found a new allele in N. bombi, the recent study by Cameron et al.
(2016) found no evidence of an exotic strain of N. bombi. While we have no documentation in
our files or evidence of direct effects of a virulent strain of N. bombi on B. franklini, N. bombi
has been detected in closely related species in the range of B. franklini. Furthermore, N. bombi
infections in rare species like B. franklini are more frequent, more severe and seem to affect a
higher percentage of individuals in the species.
Crithidia bombi has been shown to have detrimental effects on colony founding success of
queens, the fitness of established colonies, and the survival and foraging efficiency of bumble
bee workers (Brown et al. 200, p. 421; Brown et al 2003, p. 994; Otterstatter et al. 2005, p. 388;
Gegear et al. 2005, p. 1; Gegear et al. 2006, p. 1073). Studies suggest that C. bombi can spread
from commercial bumble bees to nearby wild bumble bees through shared use of flowers when
they escape to forage outside and transmit the disease (Durrer and Schmid-Hempel 1994, p. 299;
Whittington et al. 2004, p. 599; Colla et al. 2006, p. 461; Otterslatter and Thompson 2008, p. 1).
In fact, C. bombi has been shown to be present in higher frequencies in bumble bees near
greenhouses where commercial colonies of Bombus impatiens are used than in bumble bees
remote from these facilities (Colla et al. 2006, p. 621).
Although acute mortality is rarely observed, Crithidia bombi alters the foraging behavior in host
bees by reducing their ability to identify and manipulate nectar flowers. This causes bees with
high levels of infection to spend as much as 200 percent more time on flower visits to collect
pollen and nectar resources (Gegear et al. 2006, Gegear et al. 2005). Although C. bombi is
considered to be a bumble bee parasite, honey bees have also been shown to be possible vectors
(Ruiz-Gonzales and Brown, 2006, p. 621).
The extent to which this pathogen occurs within the range of Bombus franklini is not known.
However, within the historic range of B. franklini, B. impatiens hives were purchased and
installed by a strawberry and vegetable grower to pollinate their crops in Grants Pass, Oregon
(Associated Press 2007; Xerces Society and Thorp 2010, p. 18). Bombus impatiens is a known
vector of Crithidia bombi. Experimental evidence shows that bumble bees can contract C. bombi
while feeding on flowers that have been previously visited by infected bees (Tripodi, pers.
comm. 2016 in U.S. Fish and Wildlife 2016, p. 42), and bees from commercial rearing facilities
have tested positive for this pathogen upon delivery (Otterstatter et al. 2005, p. 388; Murray et
al. 2013, p. 274). While evidence exist that C. bombi does affect Bombus spp., we do not have
documentation in our files or evidence of direct effects of C. bombi on B. franklini.
Locustacarus buchneri is a tracheal mite that infects Bombus species in Japan, the Netherlands,
and Belguim. The specific effects of L. buchneri on Bombus species, as well as the mechanisms
27
for spreading the mites, are not well understood. However, Otterstlatter and Whidden (2004, p.
351) and Goka et al. (2001) cite studies that found heavy mite infestations can severely injure
bumble bees, to the extent that they are no longer able to forage (Goka et al. 2001, p. 2098).
Ottersatter and Whidden (2004) found that bumble bees containing tracheal mites have
significantly reduced lifespans in the laboratory. Commercially raised bumble bees from Europe
were found to be infested with tracheal mites at higher rates than detected in wild bees (Goka et
al. 2001, p. 2098). While evidence exists of L. buchneri effecting Bombus spp., we do not have
documentation in our files or evidence of direct effects of L. buchneri on B. franklini.
Acute Bee Paralysis was the first honey bee virus to be detected in bumble bee hosts, although its
occurrence in natural populations and effects on bumble bee health are unknown. The Black
Queen Cell Virus has been the most commonly detected bumble bee pathogen in ongoing
surveys, having been found in 31 percent of 559 samples tested to date (Tripodi, pers comm.
2016 in U.S. Fish and Wildlife Service 2016a, p. 42). It should be noted that although 12 Bombus
species across the United States have tested positive for Black Queen Cell Virus, B. franklini has
not been evaluated. The effects of this virus, which occurs not only in honey bees and bumble
bees but a number of other arthropods, are unknown (Tripodi, pers. comm. 2016 in U.S. Fish and
Wildlife Service 2016a, p. 42). We have no documentation in our files or evidence of direct
effects of acute bee paralysis on B. franklini.
Deformed wing virus (DWV) is a honey bee pathogen that results in crippled and deformed
wings. DWV was thought to only affect honey bees, until 2004, when dead Bombus terrestris
and B. pascuorum queens with deformities resembling those in honey bees were observed. Some
virus has been shown to be transmitted from honey bees to bumble bees (Singh et al. 2010, p. 1;
Furst et al., 2014, p. 3). Tripodi (pers comm. 2016 in U.S. Fish and Wildlife Service 2016a, p.
42) notes that DWV has been detected in wild and commercially-sources bumble bees. Although
virological research focuses on honey bees, many of the 24 viruses isolated from honey bees
have a broad host range, infecting some Bombus species (Manley et al. 2015, p. 2). Commercial
bumble bee producers sometimes introduce young honey bees to nesting bumble bees queens to
stimulate egg-laying, and commercially raised bumble bee colonies are often fed pollen collected
by honey bees, thus providing a potential interface that exposes bumble bees to diseases carried
by the honey bees (Genersch et al. 2006, pp. 61-62). Infected bees with deformed wings are
unable to forage. Bumble bees that were observed with deformities were also not viable
(Genersch et al. 2006, p. 61). The Petition reports of unpublished personal observations of DWV
symptoms in commercially raised B. impatiens colonies in North America, but no research is
available to determine if other species of bumble bees are also susceptible to this disease (Xerces
Society and Thorp 2010, p. 17). While evidence exist that DWV does affect Bombus spp., we do
not have documentation in our files or evidence of direct effects of DWV on B. franklini.
Notwithstanding the studies postulating Nosema bombi spillover around commercial
greenhouses (such as Colla et al. 2006, entire), as well as the timing of commercialization and
Bombus declines, Szabo et al. (2012, p. 237) found that pathogen spillover in this form cannot
fully account for the Bombus declines. Malfi and Roulston (2014, p. 24) concluded that the
evidence linking N. bombi to the Bombus declines is correlative but does suggest species
undergoing range reductions are more susceptible to N. bombi infections, while noting that it is
nonetheless possible that elevated levels of N. bombi are natural in host species. Several experts
28
have surmised that N. bombi may not be culpable (or the only culpable) pathogen in the
precipitous decline of wild Bombus in North America (e.g., D. Goulson pers. comm. 2016, J.
Strange and A. Tripodi (USDA) pers. comm. 2016 in U.S. Fish and Wildlife Service 2016a,
p.41). Cameron et al. (2011b, p. 662) sum up the likelihood of pathogen spread being a primary
cause of Bombus declines by stating that higher pathogen prevalence and reduced genetic
diversity are realistic predictors of patterns of decline in North American bumble bees, although
cause and effect remain uncertain.
Known pathogens occur within the historical range of Bombus franklini, and we have evidence
of several pathogens infecting closely related species within that range. Although we have no
direct evidence of pathogens playing a role in the decline of B. franklini, the disappearance of B.
franklini occurred soon after a period of potential exposure to introduced pathogens, particularly
Nosema bombi which is known to have a more severe impact on rare species like B. franklini.
Decline of other closely related pollinators has been associated with these pathogens and it is
highly likely the factor has had some negative influence on the health of B. franklini populations.
3.1.2 Pesticides
Bumble bee exposure to pesticides can occur from direct spray or drift (Johansen and Mayer
1990), or from gathering or consuming contaminated nectar or pollen (Morandin et al. 2005, p.
619). Lethal and sublethal effects on bumble bee eggs, larvae, and adults have been documented
for many different pesticides under various scenarios (Kevan 1975, p. 301; Johansen 1977, p.
178; Plowright et al. 1978, p. 1145; Plowright et al. 1980, p. 765; Kearns and Inouye 1997, p.
302; Kearns et al. 1998, p. 91–92; Kevan 1999, p. 378; Thompson 2001, p. 305; Gels et al. 2002,
p. 722; Morandin et al. 2005, p. 619; Mommaerts et al. 2006, p. 752; Goulson et al. 2008, pp.
11.4–11.5). Documented sub-lethal effects to individual bumble bees and colonies include
reduced or no male production (Fauser-Misslin et al. 2014, pp. 453-454; Feltham et al. 2014, p.
320; Gill et al. 2012, p. 107; Mommaerts et al. 2006, pp. 3-4; Mommaerts et al. 2010, pp. 211-
212; Scholer and Krischik 2014, p.7), reduced or no egg hatch (Elston et al. 2013, pp. 6-7;
Mommaerts et al. 2006, pp.3-4), reduced queen production (Fauser-Misslin et al. 2014, pp. 453-
454; Feltham et al. 2014, p. 320; Whitehorn et al. 2012, p. 352), reduced queen longevity
(Fauser-Misslin et al. 2014, pp. 453-454), reduced colony weight gain (Feltham et al. 2014, p.
320; Whitehorn et al. 2012, p. 351; Scholer and Krischik 2014, p. 6), reduced brood size (Elston
et al. 2013, p. 6; Feltham et al. 2014, p. 320; Gill et al 2012, p. 107; Laycock et al. 2012, p. 3),
reduced feeding (Fauser-Misslin et al. 2014, pp. 453-454; Feltham et al. 2014, p. 320; Gill et al.
2012, p. 107; Gill and Raine 2014, pp. 211-212; Scholer and Krischik 2014, p. 5; Thompson et
al. 2014, pp. 2-3), impaired ovary development (Laycock et al. 2012, pp. 4-5), and an increased
number of foragers or foraging trips or duration (interpreted as risky behaviors) (Gill et al. 2012,
p. 107; Gill and Raine 2014; pp. 5-8; Feltham et al. 2014, p. 320).
Studies have also found evidence of adverse impacts to bumble bee habitat associated with
pesticides due to changes in vegetation and the removal or reduction of flowers needed to
provide consistent sources of pollen, nectar, and nesting material (Johansen 1977, p. 188;
Plowright et al. 1978, p. 1145; Williams 1986, 54; Kearns and Inouye 1997, p. 302; Smallidge
and Leopold 1997, p. 264; Kearns et al. 1998, p. 91–92; Shepherd et al. 2003). Declines in
29
bumble bees in parts of Europe have been at least partially attributed to the use of pesticides
(Williams 1986, p. 54; Kosior et al. 2007, p. 81).
Although the use of land for agricultural purposes has traditionally involved the use of pesticides
and other products toxic to bees, one particular class of insecticides known as neonicotinoids
have been strongly implicated in the decline honey bees worldwide as well as several Bombus
species, due to the contemporaneous introduction of neonicotinoid insecticides and the
precipitous decline of those species (Pisa et al. 2015, p. 69; Goulson 2013, p. 7-8; Colla and
Packer 2008, p. 10). Neonicotinoids are based on nicotine compounds; they are systemic
insecticides that act as a neurotoxin and varying levels of toxicity, affecting the central nervous
system of insects. Laboratory data indicated that neonicotinoids kill insects by interfering with
the receptors of the insects’ nervous system, causing overstimulation, paralysis, and death. The
neonicotinoid family of insecticides includes acetamiprid, clothianidin, imidacloprid,
nitenpyram, nithiazine, thiacloprid and thiamethoxam. They are used in a wide variety of
agricultural applications.
Imidacloprid became widely used in the United States starting in the early 1990s, followed by
clothianidin and thiamethoxam in the early 2000s (Douglas and Tooker 2015, pp. 5091-5092).
As of 2013, nearly all corn planted in the United States was treated with neonicotinoids and
various fungicides (Stokstad 2013, p. 675) and as of 2014 approximately one-third of the
soybean acreage in the United States was planted with neonicotinoid-coated seeds (Douglas and
Tooker 2015, p. 5090; U. S. Geological Survey National Pesticide Synthesis 2016). Imidacloprid
is one of the most widely used insecticide in the world (Yamamoto and Casida 1999).
Most studies examining the effect of neonicotinoids on bees have been conducted using the
European honey bee (Apis mellifera), and a handful of Bombus species including B. terrestris, B.
impatiens, and B. affinis (Lundin et al. 2015, p. 7), but there have been no studies on B. franklini
(Lundin et al. 2015, p. 7). We infer, however, that studies of the effect of pesticides to other
Bombus species will likely reflect their effects on B. franklini because these species have similar
life history traits (e.g., generalist foragers collecting pollen from same food sources). Bumble
bees may, in fact, be more vulnerable to pesticide exposure than honey bees. Bumble bees are
more susceptible to pesticides applied early in the year than are honey bees, because for one
month every year the entire bumble bee population depends on the success of the queens to
forage and establish new colonies. Also, because most bumble bees have smaller colonies (N=~
several hundred to a thousand) than honey bees (N=~30,000), a single bumble bee worker is
more important to the survival of the colony than a single honey bee worker (Thompson and
Hunt 1999, p. 155; Sponsler et al.. 2017, p. 30). Furthermore, since bumble bees nest
underground, they are additionally exposed to pesticide residues in the soil, specifically when the
application of a pesticide overlaps with colony establishment in the spring (Arena and Sgolastra
2014, p. 333). Moreover, bumble bee larvae consume large amounts of unprocessed pollen, and
therefore, are much more exposed to pesticide residues in pollen (Arena and Sgolastra 2014, p.
333).
Studies (e.g., Piiroinen and Goulson 2016, entire) are now emerging that have simultaneously
documented effects to bumble bees and honey bees at field-realistic levels. As generalist
foragers, both honey bees and bumble bees are often collecting from the same pollen sources (E.
30
Evans, pers. comm. 2016, in U.S. Fish and Wildlife Service 2017, p. 45). Based on detected
concentrations in the wild and the results of toxicity test, as well as the frequency of hives across
the landscape, Sanchez-Bayo and Goka (2014, pp. 12-14) predicted that exposure to
thiamethoxam, imidacloprid, and clothianidin (along with two organophosphates--phosmet and
chloropyrifos) pose the greatest risk to honey bees at a global scale. However, the additive and
synergistic effects of exposure to multiple pesticides and multiple times may exacerbate the
toxicity of exposure to any single pesticide, and thus, additional pesticides in combination with
others, may pose risks to bees as well. Several studies have revealed that bees are often
chronically exposed to a cocktail of pesticides throughout their lifetime (Sanchez-Bayo and Goka
2014, p. 5; Chauzat et al. 2006, pp. 256-257; Mullin et al. 2010, pp. 3-8; Krupke et al. 2012, pp.
3-5). For example, Sanchez-Bayo and Goka (2014, p. 5) detected 161 different pesticides at
honey bee colonies.
The effects of chronic exposure to multiple pesticides are poorly understood and are not
regularly examined in risk assessments (Goulson 2016, p. 4), and thus, the toxicity results, may
underestimate the actual risks posed to bees. Furthermore, pesticide formulations typically
contain less than 50 percent active ingredients with the remainder being surfactants (surface
active agent that reduces the surface tension of water) and solvents (collectively, referred to as
adjuvants). As bees forage, they are exposed to many adjuvants as well as active ingredients
(Mullin et al. 2015, p. 7). Adjuvants, however, are not typically included in risk assessments that
are required for pesticide registration (Mullin et al. 2015, p. 2), and are therefore, less studied,
but can be as or more toxic to bees as the active ingredients (Mullin et al. 2015, p.4). For
example, bumble bees are highly susceptible to emulsifiers such as perfluorooctane sulfonic acid
(Mommaerts et al. 2011, pp. 450-452). Goodwind and McBrydie (2010, p.232) found that four
of 11 commercially available spray adjuvants were toxic to honey bees at field rates.
Furthermore, active ingredients and inert ingredients may interact synergistically, causing
impacts that would not occur by exposure to the active ingredients alone (Mullin et al. 2015, p.
3). Lastly, bees are exposed to a number of significant and interacting stressors (Goulson et al.
2015, entire), which can compound the effects of pesticides. Exposure to fungicides greatly
increased the toxicity of insecticides in honey bees (Schmuck et al. 2003, pp. 82-85; Iwasa et al.
2004, p. 376; Piling and Jepsen 1993, pp. 295-296; Mullin et al. 2015, p. 4). Honey bees exposed
to fungicides had reduced colony nutrition and higher virus levels to fungicides (DeGrandi-
Hoffman et al. 2015, pp. 2523-2524). Pettis et al. (2013, p. 4), for example, found increased
probability of Nosema infection in honey bees feeding on pollen with high fungicide loads.
Several studies found exposure to insecticides reduced resistance to diseases (Fauser Misslin et
al. 2014, pp. 454-455, Pettis et al. 2013, p. 4), and exposure to dietary related stresses (e.g.,
short-term starvation) reduced the ability of bees to cope with toxins (Brown et al. 2000, p. 424;
Tyler et al. 2006, p. 2; Moret and Hempel 2000, p. 1167). Piiroinen and Goulson (2016, pp. 3-5)
found that exposure to N. ceranae reduced learning in honeybees and bumble bees, but both
species reacted differently to the combination of pathogen plus pesticide exposure.
Determining the extent of bee fatality caused by pesticides is difficult due to the myriad of other
potential stressors (e.g., pathogens, parasitoids, and diseases) and possible synergistic effects of
these sources. There are known instances where neonicotinoids such as clothianidin have adverse
effects to immunity and promote replication of viral pathogens in bees (e.g., DiPrisco et al. 2013,
31
p. 3). The interruption or disruption of endocrine functions is related to the function of species’
immune systems and the application of neonicotinoids may exacerbate the effects of pathogens.
To assess the perceived cause and effect relationship between neonicotinoid application levels
and Bombus franklini declines, we gathered available data on pesticide use for a subset of
chemicals and charted the application trend over time throughout the range of B. franklini.
Specifically, using pesticide application rate data collected from 1995 to 2015 (United States
Geological Survey National Pesticide Synthesis, accessed November 2017), we examined the
trend in use of three prevalent neonicotinoids; imidacloprid, clothianidin, and thiamethoxam over
time in 5 counties with recent (since 1995) B. franklini occurrences. Limited information on
neonicotinoid application in California is available in this dataset, so in addition we received data
from the California Pesticide Information Portal (accessed December 2017). All three chemicals
were added for each year to get a total application rate of imidacloprid, clothianidin, and
thiamethoxam combined. While we chose to focus these trend analyses on three commonly used
and studied neonicotinoids, we recognize that there are a myriad of pesticides, inactive
ingredients, and other chemicals that have documented negative effects on bees (as discussed
above) and could be similarly analyzed for application rate trends in our study area. Furthermore,
the vast majority of neonicotinoids are used as seed treatments on grains and other field crops
(Oregon Department of Agriculture 2018, pers. comm.). The National Pesticide Synthesis data
are both a high and low estimate of application rates based on the best available data – see
Appendix 3 for more information on the sources, assumptions, and use limitations of the data.
We also recognize that the timing, location, and methods of pesticide application play a role in
their effectiveness on target species (i.e., aerial spraying of row crops vs. placement of ant traps).
For our study area (Jackson, Douglas, and Josephine Counties in Oregon as well as Trinity and
Siskiyou Counties in California), the first reported use of Imidacloprid was in 1996;
thiamethoxam first reported in 2001, and clothianidin in 2004. Total estimated neonicotinoid
applications increased from 53.35 pounds/acre (24.19 kilograms/acre) in 1996 to 1,144.128
pounds/acre (518.86 kilograms/acre) in 2014.
While the rapid decline of Bombus franklini observations occurred shortly after the introduction
of neonicotinoid pesticide use within the historic range of the species, the exponential growth of
neonicotinoid applications starting in 2011 took place five years after the last observation of the
species so it is unlikely that the introduction and use of neonicotinoid pesticides alone can
account for the decline in B. franklini. There have been no studies on the effects of pesticide use
on B. franklini, no documented discoveries of any B. franklini injured or killed by pesticides.
Furthermore, the species is a habitat generalist and is not known to have a close association with
agricultural lands so it may have less exposure to pesticides than some other Bombus species.
However, pesticide use does occur in the range of B. franklini and confirmed effects to honey
bees and other Bombus species suggests that pesticide use could have been a factor in the decline
of B. franklini. The similarity in foraging traits that B. franklini has with both honey bees and the
other Bombus species (e.g., generalist foragers collecting pollen from similar food sources)
allows us to infer that that B. franklini would suffer exposure to and impacts from pesticides in
similar measure to other Bombus species when B. franklini is in areas where pesticides are
applied.
32
3.1.3. Habitat Loss and Degradation
Habitat loss and degradation was identified by the Petitioners as a threat contributing to the
decline of Bombus franklini (Xerces Society and Thorp 2010, p. 4). Habitat loss and degradation
can be manifest in many forms over different spatial and temporal scales. In this section we look
at habitat loss and degradation in the range of B. franklini and its potential effects on the species
through agricultural intensification, natural and introduced fire, and urbanization; livestock
grazing and climate change are discussed later in separate sections.
Conversion of natural habitat that is rich in flowers to farmlands, urban and suburban areas, and
other uses is a primary cause of bumble bee habitat loss (Goulson et al. 2015, p.2). Agricultural
intensification can result in habitat loss for bumble bees, as these practices often result in the
planting of monocultures, which tend to provide floral resources for a limited period of time,
rather than throughout the colony life cycle. Studies have confirmed that agricultural
intensification can negatively impact wild bees by reducing floral resource diversity and
abundance (Johansen 1997, p. 177; Williams 1986, p. 57; Kearns et al. 1998, p. 89; Hines and
Hendrix 2005, p. 1477; Carvell et al. 2006, p. 481; Diekotter et al. 2006, p. 57; Fitzpatrick et al.
2007, p. 185; Kosior et al. 2007, pp. 81, 84-86; Ockinger and Smith 2007, pp. 50; Goulson et al.
2008, p. 11.1; International Union for Conservation of Nature 2009, p. 2; Le Feon et al. 2010, p.
143). Agricultural intensification was determined to be a primary factor leading to the local
extirpation and decline of Illinois bumble bees (Grixti et al. 2009, p. 75). An increase use of
herbicides often accompanies development and agricultural intensification, and the wide-spread
use of herbicides in agricultural, urban and even natural landscapes has led to decreases in
flowering plants (Potts et al. 2010, p. 350).
Douglas, Jackson and Josephine Counties in Oregon, and Siskiyou and Trinity Counties in
California, are generally characterized as rural, agriculturally based counties with large
proportions of public land and which lack the larger population centers found to the north and
south of the historical range of Bombus franklini. Information specific to agricultural
intensification within the historic range of B. franklini is not available at the spatial and temporal
scales needed to quantify this threat (United States Department of Agriculture – National
Agriculture Statistics Service, pers. comm. 2017). However, between 1997 and 2012, Oregon
saw a decrease in both the overall number of farms and ranches, as well as a decrease of more
than a million acres of land in agriculture (United States Department of Agriculture – National
Agriculture Statistics Service 2015, p. 6). Within the historic range of B. franklini, Douglas,
Jackson and Josephine Counties all saw a similar decrease of total acres in agricultural cropland,
as summarized in Table 4 below (United States Department of Agriculture – National
Agriculture Statistics Service, pers. comm. 2017). While the total number of acres of agricultural
cropland is not synonymous with agricultural intensification (specifically, the expansion of
monocultures), a decrease in total acres of agriculture leads us to conclude that agricultural
intensification is not likely a major threat to B. franklini. We have no documentation in our files
or any direct evidence that agricultural intensification has contributed to the decline of B.
franklini.

33
Table 4. Acres of Agricultural Cropland in Douglas, Jackson, and Josephine Counties in Oregon
Data: USDA-NASS 2015, 2017
Acres of Agricultural Cropland
1997
2002
2007
2012
Douglas
123,133
107,503
73,559
49,222
Jackson
71,251
67,762
56,530
32,765
Josephine
17,767
15,860
17,389
8,365
Oregon
1997
2002
2007
2012
Number of farms and ranches
39,975
40,033
38,533
35,439
Total land in agriculture
(millions of acres)
17.7
17.2
16.4
16.3
Forty-two percent of the sites where Bombus franklini have been located (18 of 43) occur on
federally owned land, primarily the U.S. Forest Service and Bureau of Land Management. This
notable proportion could be due to a good percentage of B. franklini occurring on Federal land or
simply that searches for B. franklini often occur on Federal land. Very little habitat on these
ownerships has been permanently altered or lost through development or agricultural
intensification.
Fire caused by both natural and manmade factors has been an important change agent on the
landscape in the range of Bombus franklini. Because fire reduces natural succession of forests
through the burning of encroaching woody plants, fire is a primary factor in the maintenance of
grassland and meadow habitat that supports Bombus species (Shultz and Crone 1998, p. 244;
Huntzinger 2003). With the increase in human development came fire suppression to limit
damage to manmade structures. Fire suppression allows woody encroachment to occur and the
diverse landscape created by fire (open areas mixed within forested areas) is being replaced by
increasing areas of denser forested habitat; the open areas that facilitated the growth of diverse
understory plant communities are greatly reduced from their historical condition (Ruchty 2011,
p. 26). Conifer species now cover much of the area that was previously open meadow habitat in
the range of B. franklini (Panzer 2002; Shultz and Crone 1998, p. 244). This loss of habitat by
fire suppression likely played some role in the decline if B. franklini by limiting the availability
and diversity of floral resources and nest and overwintering habitat. However, because there is
still healthy meadow habitat located in areas where B. franklini were previously found, we do not
believe that loss of habitat from fire suppression was a major factor in the decline of the species,
particularly the precipitous decline that occurred after 1998.
The increased fuel loads from fire suppression increase the potential for catastrophic, large scale,
and high temperature wildfires. Any Bombus colonies in the path of this type of fire would be at
risk of extirpation. Wildfire may have played a role in the decline of B. franklini by extirpating
some historical populations in the range, but we have no information confirming this. We have
no information that suggests that any known B. franklini occurrence sites were in the path of
catastrophic wildfires at the time they were occupied. Controlled burning became a management
tool for reducing potential fuel loads for wildfire; controlled burning and other fuel reduction
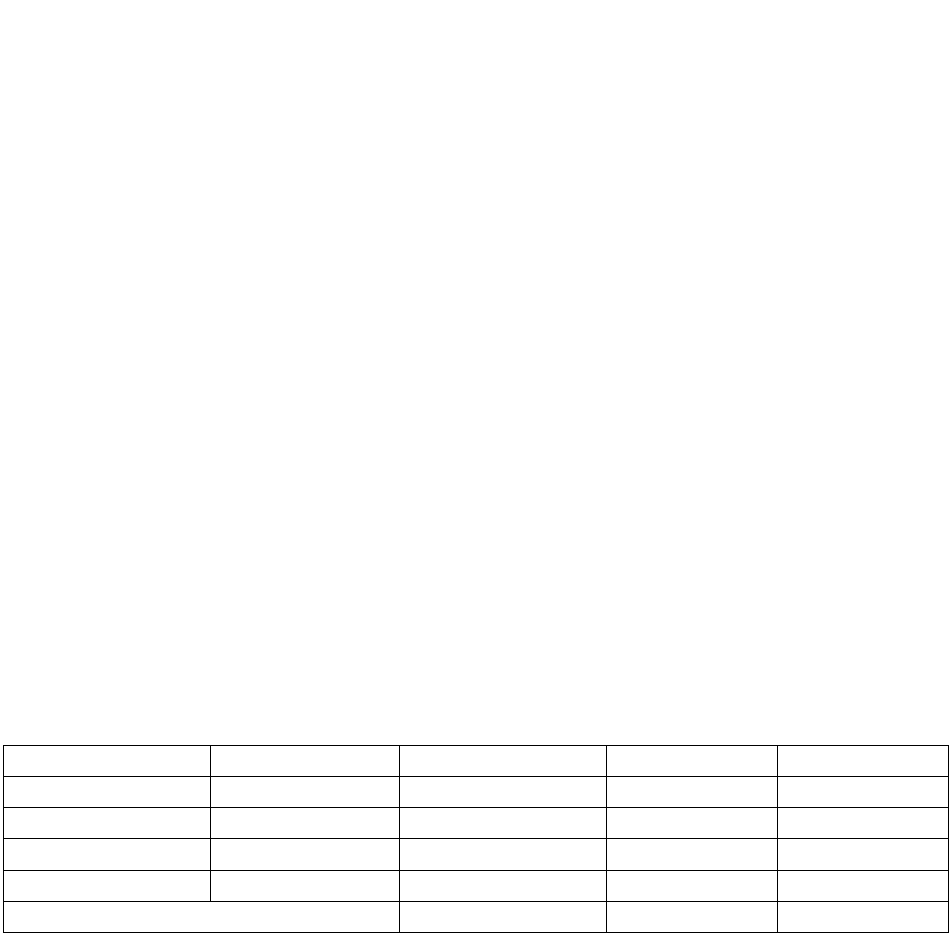
34
activities are carried out by Federal land management agencies including the US Forest Service
and Bureau of Land Management in the range of B. franklini. The effects of fire on invertebrates
depends greatly on the biology of the specific taxa (Gibson et al. 1992) and in the case of B.
franklini, controlled burns could certainly cause death of individual bees and negative effects to a
colony. However, we have no information to indicate that controlled burns were a factor in the
decline of B. franklini.
Ongoing urbanization also contributes to the loss and fragmentation of natural habitats. Urban
gardens and parks may provide habitat for some pollinators including bumble bees (Frankie et al.
2005, McFrederick and LeBuhn 2006) but they tend not to support the species richness of
bumble bees that can be found in nearby undeveloped landscapes (Xerces Society and Thorp
2010, p. 13), or that which was present historically (McFrederick and LeBuhn 2006). Bombus
franklini and B. occidentalis have both been observed in urban areas of Ashland, Oregon, but not
since 2002. A study in Boston, Massachusetts, concluded that human built structures, such as
roads and railroads, can fragment plant populations and restrict bumble bee movement
(Bhattacharya et al. 2003, p. 37). Urban development may also lead to direct mortality, i.e.
through vehicle collisions (Goulson et al 2015, p. 2). Another study of the factors adversely
affecting bumble bees and cuckoo bees in Europe found the expansion of urban areas to be an
important driver of pollinator loss in approximately half of the countries examined (Kosior et al.
2007, p. 81).
Table 5 shows the 1995 and 2017 populations for Douglas, Jackson, and Josephine Counties, as
well as the population for Ashland, OR. Table 5 also shows the population growth estimates that
were completed in 2015 for each of the counties.
Table 5. Human population growth estimates for Douglas, Jackson, and Josephine Counties in
Oregon and Ashland, Oregon.
1995 population
2017 population*
2035 estimate
2065 estimate
Douglas County
98,820
111,180
129,910
152,910
Jackson County
167,330
216,900
246,575
306,575
Josephine County
71,290
85,650
99,720
121,720
Ashland, Oregon
17,985
20,700
*2017 data preliminary
For Bombus in general, loss and degradation of habitat is known to reduce both bee diversity and
abundance (Potts et al. 2010, p. 348-349). Habitat fragmentation can alter pollinator community
composition, change foraging behavior of bumble bees, and reduce bee foraging rates, and is
believed to be one of the factors contributing to the decline of several bumble bee and cuckoo
bee species in Europe (Kearns and Inouye, 1997, p. 299; Ockinger and Smith 2007, p. 50;
Rusterholz and Baur 2010, p. 148; Kosior et al 2010, pp. 81). Bumble bees have been found to
be susceptible to the disruption of healthy metapopulation structures due to fragmentation, and
may decrease source populations of bumble bees for recolonization (National Research Council
2007, p. 93; Goulson et al. 2008, p. 11.7). Other studies have suggested that fragmented bumble
bee populations can suffer from inbreeding depression as a result of geographic isolation (Darvill
et al. 2006, p. 601, Goulson et al. 2008, p. 11.7) (see sections 2.2.2 and 3.1.6 for more on genetic
impacts from small population sizes).
35
Although habitat loss and fragmentation has established negative effects on bumble bees
(Goulson et al. 2008; Williams and Osborne 2009, pp. 371-373), many feel it is unlikely to be a
main driver of the recent, widespread North American bee declines (Szabo et al. 2012, p. 236;
Colla and Packer 2008, p. 1388; Cameron et al. 2011b, p. 665). Further, habitat remains
generally intact and in good condition throughout the known historic Bombus franklini locations
and all of the recent focused survey areas, with the notable exceptions being the creation of Lake
Applegate upon the completion of Applegate Dam in the fall of 1980 and a report of soil
modification on a portion of the Gold Hill site. The Applegate Dam project inundated two
historic B. franklini locations (Copper and 2 miles north of Copper), with historic observations
from 1963 and 1968 (Xerces Society and Thorp 2010, p. 13; Thorp, pers. comm. 2017). The
Petition noted that in 2004, soil had been excavated and deposited in a portion of the Gold Hill
area (Xerces Society and Thorp 2010, p. 13). The last observation of B. franklini at Gold Hill
was in the year 2000, and the site was revisited 14 times over the next three years with no
observations of the species. At both Lake Applegate and Gold Hill, we don’t know if the species
was still using the habitat by the time the activities took place. Overall, many feel that habitat
loss and fragmentation are not a main driver of the decline of B. franklini, particularly since
many other Bombus species have been recorded in the habitat where searchers have looked for B.
franklini (Thorp, pers. comm. 2017; Godwin, pers. comm. 2017; Colyer, pers. comm. 2017).
3.1.4 Livestock Grazing
Livestock grazing occurs on public land on much of the historic range of Bombus franklini. The
Petition stated that livestock grazing may adversely impact bumble bee populations by: (1)
depleting food resources (Morris 1967, p. 472; Sugen 1985, p. 299; Kruess and Tscharntke
2002b, p. 1570; Vazquez and Simberloff 2003, p. 1081; Hatfield and LeBuhn 2007, p. 150); (2)
trampling nesting sites (Sugden 1985, p.299); and (3) negatively impacting ground-nesting
rodents (Johnson and Horn 2008, p. 444; Schmidt et al. 2009, p. 1), which may in turn reduce the
number of nest sites available for bumble bees (Xerces Society and Thorp 2010, p. 13). The
Petition also stated that livestock grazing has differing impacts on flora and fauna based on the
type, habitat, intensity, timing and length of grazing (Gibson et al. 1992, p. 174; Carvell 2002, p.
44; Kreuss and Tscharntke 2002a, p. 293; Kruess and Tscharntke 2002b, p. 1577; Xerces Society
and Thorp 2010, p. 13). Several studies of livestock grazing impacts on bees suggest increased
intensity of livestock grazing affects the species richness of bees (Morris 1967, p. 473; Sugden
1985, p. 309; Vazquez and Simberloff 2003, p. 1080; Hatfield and LeBuhn 2007, p. 156). In
contrast, one study cited in the Petition suggests that grazing, especially by cattle, can play a key
positive role in maintaining the abundance and species richness of preferred bumble bee forage
(Carvell 2002, p. 44).
Overgrazing by sheep between 1890 and 1920, resulted in trampling vegetation and denuding
soils, and is currently evident today in the continuing erosion of the granitic soils of the
McDonald Basin, Siskiyou Gap, Mt. Ashland, and the Siskiyou Crest (LaLande 1995, p. 31; T.
Atzet, Siskiyou Field Institute, Selma, Oregon, pers. comm. 2017). While sheep overgrazing
likely has degraded B. franklini habitat, we have no specific information on the effects of this
habitat loss and fragmentation. Evidence of livestock grazing has been observed interspersed
within abundant floral resources in B. franklini habitat during several recent targeted survey
efforts (Brooks 1997 pers. comm.; U.S. Fish and Wildlife Service 2016; U.S. Fish and Wildlife
36
Service 2017; P. Trail, U. S. Fish and Wildlife Service, Ashland, Oregon, pers. comm. 2017).
However, no specific information is available on the impacts of livestock grazing on B. franklini
making it impossible to connect the activity to any specific species response. The number of
grazing allotments have decreased on The Rogue-River Siskyou NF, particularly on the Siskiyou
Mountains Ranger District (Applegate) in the last 20 years; the grazing on High Cascades
Ranger District (near Prospect, OR), have not changed in the last 20 years (J. von Kienast, pers.
comm. 2018). Generally the dates that cows are on the allotments start on June 15
th
and stay on
until October but dates vary by allotment. Most of the locations for B. franklini on the Rogue
River-Siskiyou NF overlap with grazing allotments. Cattle grazing has been observed at all
Bombus survey locations on the High Cascades Ranger District. (S. Colyer pers. comm. 2018).
Outside of these areas, we have no new information that the timing, location, intensity, or
duration of grazing has changed, with the exception of the Cascade-Siskiyou National
Monument, where most grazing has been retired on the Cascade-Siskiyou National Monument
(Trail, pers. comm. 2017) (See Figure 1).
3.1.5 Climate Change
Global climate change was identified in the Petition as a threat to Bombus franklini (Xerces
Society and Thorp 2010, pp. 20-21). Climate change may cause shifts in the range of host plant
species, which can be especially detrimental to dependent pollinators when combined with
habitat loss (Xerces Society and Thorp 2010, p. 20; Schroeder, pers. comm. 2017). Specific
impacts of climate change on pollinators are not well understood; most of the existing
information on climate change impacts to pollinators comes from studies on butterflies – studies
specifically relating to bumble bees are scant, and we found no climate change information
specific to B. franklini.
Climatic changes in temperature, precipitation, and the increased frequency of storm events
(Intergovernmental Panel on Climate Change 2013, entire) can affect population sizes directly,
affecting survival and reproduction (Bale et al. 2002, p. 11; Roland and Matter 2016, p. 22).
These climatic changes can also affect populations indirectly, by altering resource availability
and species interactions (Boggs and Inouye 2012, p. 505; Hoye et al. 2013, p. 762; Kudo and Ida
2013, p. 2319). Some studies suggest that bumble bee populations are responding to climate
change with recent latitudinal and elevational range shifts (Ploquin et al 2013, p. 9; Pyke et al
2016, p. 11). Some bumble bee populations are active earlier in the season than in the past
(Bartomeus et al 2011, p. 20646). Ogilvie et al. (2017, p. 1) found that bumble bee abundances
were driven primarily by the indirect effects of climate on the temporal distribution of floral
resources.
The changes in climate likely to have the greatest effects on bumble bees in general include
increased drought, increased flooding, increased storm events, increased temperature and
precipitation events, early snow-melt, late frost, and increased variability in temperature and
precipitation. These climate changes may lead to decreased resource availability (due to
mismatches in temporal and spatial co-occurrences), decreased availability of nesting habitat
(due to higher temperatures), and increased pressures from pathogens and non-native species
(Goulson et al. 2015, p. 4; Goulson, pers. comm. 2016 in U.S Fish and Wildlife 2016, p. 52; Kerr

37
et al. 2015, pp. 178-179; Potts et al. 2010, p. 351; Cameron et al. 2011a, pp. 35-37; Williams and
Osborne 2009, p. 371).
Climate variability may lead to range shifts, such that there is spatial mismatch among plants and
their pollinators (Memmott et al. 2007, p. 712). While this has been demonstrated in butterflies
(Forister et al. 2010, pp. 2088-2089; Hickling et al. 2006, p. 452), it may be less of a factor for
bumble bees. As generalist foragers, they do not require synchrony with a particular plant
species. However, elevational range shifts have been documented in some bumble bees (e.g.,
Pyke et al. 2016, pp. 8-10; Kerr et al 2015, p. 179). Temporal mismatches may be more of an
issue for bumble bees due to their long active season, during which they require consistent access
to floral resources. Floral resource availability in early spring is particularly crucial for bumble
bees, as that is when they first emerge and initiate nests. Thus, temporal asynchrony could lead
to diminished resource availability at times that are critical to bee development and colony
success. Other potential effects from climate change include increased flooding and storm
events, which may directly reduce available nesting habitat and hibernating habitat by inundating
those areas (Goulson et al. 2015, p. 4). Changes in rodent populations due to climate change may
also reduce nesting habitat, as bumble bees often use rodent burrows as nesting areas.
Furthermore, bumble bees are poorly adapted to high temperatures, and thus are vulnerable to
increased stress from overheating.
Several of the targeted Bombus franklini and B. occidentalis survey reports between 2015 and
2017 include mention of widespread hot, dry climate affecting timing and abundance of floral
resources during the surveys (Bureau of Land Management 2015; Trail, pers. comm. 2017),
indicating that at least at local scales in recent years, changing climate conditions may have
affected resources available to Bombus colonies. Although the Olgilvie et al. study as well as the
survey reports suggest potential indirect effects of climate change on Bombus, we have no
information to indicate that the effects of climate change were connected to the decline of B.
franklini; numerous Bombus species persist in areas considered to maintain good quality habitat
for B. franklini (Pool 2014, entire; Colyer 2016, entire).
3.1.6 Small Population Dynamics
Small population size has been identified as a threat to Bombus franklini. The Petition (Xerces
Society and Thorp, p. 20) states that B. franklini is rare and has always had very small
populations (relative to other similar, native bumble bees in the western United States), and
likely have low genetic diversity, making the species more vulnerable to habitat change or loss,
parasites, diseases, stochastic events, and other natural disasters such as droughts (Xerces
Society and Thorp 2010, p. 20).
As stated in section 2.1.2, between 1998 and 2006, the number of Bombus franklini observations
declined from a high of 98 at 8 locations, to a lone individual in 2006. No observations of B.
franklini have occurred since 2006 despite an increase in the survey effort.
As mentioned in section 2.2.2, bumble bees exhibit a haplodiploidy sex determination system. In
these systems, unfertilized (haploid) eggs become males that carry a single set of chromosomes,
and fertilized (diploid) eggs become females that carry two sets of chromosomes. This may

38
result in lower levels of genetic diversity than the more common diploid-diploid sex
determination system, in which both males and females carry two sets of chromosomes.
Haplodiploid organisms (such as bumble bees) may be more prone to population extinction than
diploid-diploid organisms, due to their susceptibility to low population levels and loss of genetic
diversity (Packer and Owen 2001, p. 26; Zayed and Packer 2005, p. 10742; Darvill et al. 2006, p.
601, Ellis et al. 2006, 4375; Goulson et al. 2008, p. 11.7-11.9). Inbreeding depression in bumble
bees can led to the production of sterile diploid males (Goulson et al. 2008, p. 11.7) and has been
shown to negatively affect bumble bee colony size (Herrman et al. 2007, p. 1167), which are key
factors in a colony’s reproductive success. Diploid male production has been detected in
naturally occurring populations of bumble bees, and recent modeling work has shown that
diploid male production, where present, may initiate a rapid extinction vortex (a situation in
which genetic traits and environmental conditions combine to lead a species to extinction)
(Goulson et al. 2008, p. 11.8). Bombus franklini is a haplodiploid organism with a relatively
small population size compared to other Bombus species. A haplodiploid genetic system makes
bees very vulnerable when populations get small because of inbreeding and the production of
sterile males (Colla, 2018, pers. comm.). Although we have no direct evidence that small
population size or a rapid extinction vortex contributed to the decline of the species, the genetic
system and historically small population size of B. franklini likely heightened the species’
vulnerability to other stressors in the environment.
3.1.7 Competition from non-native bees
The European honey bee (A. mellifera), was first introduced to eastern North America in the
early 1620s, and introduced to California in the early 1850s (Xerces Society and Thorp, p.21).
The resources of A. mellifera and native Bombus species may overlap resulting in the potential
for increased competition for resources (Thomson 2004, p. 458; Thomson 2006, p. 407;
Thomson 2016, p. 1247). Decreased foraging activity and lowered reproductive success of
Bombus colonies have been noted near A. mellifera hives (Evans 2001, p. 32–33; Thomson 2004,
p. 458; Thomson 2006, p. 407). Additionally, the size of workers of native Bombus species were
noticeably reduced where A. mellifera were present, which may be detrimental to Bombus colony
success (Goulson and Sparrow 2009, p. 177). As noted in the 2010 Petition, is likely that the
effects discussed in these studies are local in space and time, and most pronounced where floral
resources are limited and large numbers of commercial A. mellifera colonies are introduced
(Xerces Society and Thorp, p. 21). We could not find information to indicate that any area of B.
franklini habitat in the range of the species has limited floral resources and large numbers of A.
mellifera. We have no information related to the specific placement of commercial honey bee
colonies in or near B. franklini habitat. Furthermore, A. mellifera have been present without
noticeable declines in Bombus populations over large portions of their ranges (Xerces Society
and Thorp, p. 21) and we have no new information that connects competition from A. mellifera
to the decline of B. franklini, particularly the noticeable decline after 1998.
There is potential for non-native commercially raised bumble bees to naturalize and outcompete
native bumble bees for limited resources such as nesting sites and forage areas. Five
commercially reared Bombus impatiens workers and one queen were captured in the wild near
greenhouses where commercial bumble bees are used, suggesting this species may have
naturalized outside of its native range. In this study, B. impatiens, which has a native range in

39
eastern North America, was detected in western Canada (Ratti and Colla 2010, pp. 29–31). A
study in Japan found that non-native B. terrestris colonies founded by bees that had escaped
from commercially produced colonies had over four times the mean reproductive output of
native bumble bees (Matsumura et al. 2004, p. 93). A study in England found that commercially
raised B. terrestris colonies had higher nectar-foraging rates and greater reproductive output than
a native subspecies of B. terrestris (Ings et al. 2006, p. 940). The 2010 Petition noted that B.
impatiens colonies were imported to pollinate agricultural crops and strawberries in Grants Pass,
Oregon, in the range of B. franklini (Xerces Society and Thorp, p. 18). Although non-native
Bombus species in the range of B. franklini could outcompete B. franklini for floral resources and
nesting habitat, we could not find any information to definitely connect competition with non-
native bumble bees to the decline of B. franklini. Furthermore, invertebrate surveys in B.
franklini habitat continue to show evidence of healthy populations of other native Bombus
species unaffected by competition from non-native bees (Pool 2014, entire; Colyer 2016, entire).
3.2 Synergistic Effects
It is likely that several of these risk factors are acting additively and synergistically on Bombus
species (Goulson et al. 2015, p. 5) and the combination of multiple stressors is likely more
harmful than a stressor acting alone (Gill et al. 2012; Coors and DeMeester 2008; Sih et al.
2004). There is recent evidence that the interactive effects of pesticides and pathogens could be
particularly harmful for bumble bees (Fauser-Misslin et al. 2014, pp. 453-455; Baron et al. 2014,
pp. 463-465) and other bees (Alaux et al. 2010, pp. 775-777; Pettis et al. 2012, pp. 155-156;
Vidau et al. 2011, pp. 3-5; Aufavre et al. 2012, pp. 2-3). Nutritional stress may compromise the
ability of bumble bees to survive parasitic infections as evidenced by a significant difference in
mortality in bumble bees on a restricted diet than well fed bees infected with Crithidia bombi
(Brown et al. 2000, pp. 424-425). Bumble bees with activated immunity may have metabolic
costs, such as increased food consumption (Tyler et al. 2006, p. 2; Moret and Schmid-Hempel
2000, pp. 1166-1167). Additionally, exposure to pesticides may increase with increased food
consumption in infected bees (Goulson et al. 2015, p. 5). There is evidence that activating
immunity impairs learning in bumble bees (Riddelland Mallon 2006, Alghamdi et al. 2008, p.
480). Impaired learning is thought to reduce the ability of bees to locate floral resources and
extract nectar and pollen, therefore, exacerbating nutritional stresses (Goulson et al. 2015, p. 5).
Further, evidence of the relationship between low genetic diversity and disease susceptibility was
discussed in Cameron et al. (2011b, p. 665), who stated that declining North American species
with low genetic diversity have higher prevalence of the pathogen N. bombi. Therefore,
pathogens in combination with pesticides, and pathogens in combination with the effects of small
population size likely hastened and amplified the decline of B. franklini to a greater degree than
any one of the three factors would cause on its own.
3.3 Beneficial Actions
We are aware of no conservation efforts or beneficial actions specifically taken to address the
stressors to Bombus franklini. Oregon does not include invertebrates on their state endangered
species list (ODFW 2018) and California has no bees on its list of Threatened and Endangered
Invertebrates (CDFW 2018). California has the species listed on its list of Terrestrial and Vernal
40
Pool Invertebrates of Conservation Priority but has no required actions or special protections
associated with the listing (CDFW 2017, p. 10). Bombus franklini is on the species index for the
U.S. Forest Service and Bureau of Land Management Interagency Special Status /Sensitive
Species Program (ISSSSP) (ISSSSP 2018). Though the agencies do include the species in survey
efforts and conduct general meadow enhancement activities like reducing conifer encroachment,
there are no actions resulting from the ISSSSP classification that reduce or ameliorate known
threats to B. franklini.
The U.S. Forest Service is working to include a section in all biological evaluations to address
the effects from agency actions on pollinators. In addition, the Rogue River-Siskiyou National
Forest is currently implementing projects and mitigations to create and enhance pollinator habitat
(S. Colyer, pers. comm. 2018) The Oregon Department of Agriculture restricts some potential
sources of N. bombi from entering the state for agricultural uses, including commercially-
produced colonies of Bombus impatiens; only Bombus species native to Oregon are permitted for
commercial pollination purposes (Oregon Department of Agriculture 2017, p. 5). California does
however allow for the importation of B. impatiens, and other species such as the Blue Orchard
Bee (Osmia lignaria) for pollination services with appropriate permits in both Oregon and
California (California Department of Food and Agriculture 2017; Oregon Department of
Agriculture 2017).
General awareness of honey bee colony losses and increase of conservation efforts for
pollinators in general has likely had limited, indirect effects. Stemming from this general
awareness is a reduction in the use of some pesticides throughout North America. Some local
municipalities have enacted legislation against aerial pesticide applications but similar efforts
have not been adopted at the state or range-wide scales (Powell 2017, p. 1; City of Portland
2015, p. 2). However, in the 2017 legislative session, Oregon passed an Avoidance of Adverse
Effects on Pollinating Insects law (ORS 634.045) that is providing enhanced training of licensed
and unlicensed pesticide applicators in the state (A. Melathopoulos, pers. comm. 2018). In
January 2017, the U.S. Environmental Protection Agency’s Office of Pesticide Programs
published their Policy to Mitigate the Acute Risk to Bees from Pesticide Products, which
recommended new labeling statements for pesticide products including warnings for pesticides
with a known acute toxicity to bees including neonicotinoids (specifically including
imidacloprid, clothianidin, and thiamethoxam) (United States Environmental Protection Agency
2017, p. 31). In addition, EPA is working with state and tribal agencies to develop and
implement local pollinator protection plans, known as Managed Pollinator Protection Plans
(MP3s). EPA is promoting MP3s to address potential pesticide exposure to bees at and beyond
the site of the application. However, states and tribes have the flexibility to determine the scope
of pollinator protection plans that best responds to pollinator issues in their regions. For example,
state and tribal MP3s may address pesticide-related risks to all pollinators, including managed
bees and wild insect and non-insect pollinators (United States Environmental Protection Agency
2018).
4.0 Analysis of Current Condition
As described in section 1.2, we applied the conservation biology principles of resiliency,
representation, and redundancy (the 3Rs) as a framework to assess the viability of Bombus
41
franklini. For a species to sustain populations over time it needs a sufficient number and
distribution of viable populations to withstand environmental stochasticity (resiliency),
catastrophes (redundancy), and changes in its environment (representation). To assess resiliency
and redundancy, we evaluated the change in B. franklini occurrences (populations) over time. To
assess representation (as an indicator of adaptive capacity) of B. franklini, we evaluated the
spatial extent of occurrences over time.
Resiliency is the ability to sustain populations in the face of environmental variation and
transient perturbations. In section 2.2.3 we described that Bombus franklini requires the
following for resiliency: (1) populations with large N
e
, (2) sufficient floral resources in close
proximity to nesting and overwintering sites, (3) connectivity among colonies and populations,
and (4) spatial heterogeneity. Historically, the species has always been rare and has one of the
narrowest distributions of any Bombus species in the world. Even so, the abundance and
distribution of B. franklini has declined significantly (U.S. Fish and Wildlife Service 2018, pp.
10-14); the species has not been observed since 2006 despite an intensive survey effort in some
areas of the historical range. Prior to 1998, search efforts for the species were varied in timing,
scope, intensity, and methodology. During the more intensive surveys from 1998 until the last
observation in 2006, B. franklini was observed at 11 sites, including seven locations where it had
not been previously documented. In 1998, 98 bees were found among eight locations. Searchers
found fewer and fewer bees after that even though they continued extensive searches in multiple
locations with the highest likelihood of finding the species. Twenty bees were located in 1999,
nine individuals were observed in 2000, and one individual in 2001. Although 20 B. franklini
were observed in 2002, only three were observed in 2003 (all at a single locality), and a single
worker bee was observed in 2006. Despite continued intensive search efforts through 2017, there
have been no confirmed observations of B. franklini since 2006. There are currently no known
healthy B. franklini individuals and therefore no known healthy colonies or populations of B.
franklini. Despite the fact that some high quality habitat with diverse floral resources and
available nesting and overwintering sites appears to be available in the historic range of B.
franklini, no individuals of the species have been found in any habitat since 2006. The resiliency
of B. franklini has declined significantly since the late 1990’s.
Representation is the ability to adapt to changing environmental conditions; it is the species’
evolutionary capacity or flexibility. In section 2.2.3 we described that Bombus franklini requires
the following for representation: healthy populations distributed across areas of unique adaptive
diversity (i.e., ecoregions) to maintain evolutionary drivers (gene flow, natural selection, genetic
drift) to mimic historical patterns. Bombus franklini is rare and has always had very small
populations (relative to other similar, native bumble bees in the western United States), and
likely have low genetic diversity, making the species more vulnerable to environmental factors.
As a haplodiploid organism, B. franklini may be more prone to population extinction than
diploid-diploid organisms, due to its susceptibility to low population levels and loss of genetic
diversity. No B. franklini have been observed since 2006 despite an intensive survey effort and
therefore we cannot identify any current populations of B. franklini distributed across any level
of ecological conditions. The vulnerability resulting from B. franklini’s genetic system and the
loss in the spatial extent of its populations suggest the representation of B. franklini has declined
significantly since the late 1990’s.

42
Redundancy protects species against the unpredictable and highly consequential events for which
adaptation is unlikely. In section 2.2.3 we described that Bombus franklini requires the following
for redundancy: sufficient distribution to guard against catastrophic events wiping out portions of
the species adaptive diversity, i.e., to reduce covariance among populations, and an adequate
number of healthy populations to buffer against catastrophic losses of adaptive diversity. Bombus
franklini has the smallest geographic distribution of any North American bumble bee and
possibly the world (Williams 1998, as cited in Xerces Society and Thorp 2010, p. 6), and thus
likely had low redundancy prior to its decline. When we look at occurrence data for the species
and overlay it with our 6 km
2
grid estimating minimum habitat patch to estimate the number of
populations present on the landscape, we find that each site where B. franklini has ever been
observed could potentially reflect a population. Therefore, data allow us to estimate 43 potential
populations of B. franklini since records have been kept. From 1998 to 2006, 14 potential
populations could be identified and no B. franklini have been observed since 2006 despite a more
intensive survey effort in some areas of the historic range. We cannot identify any current
healthy populations distributed across any spatial extent. The losses in both the number of
populations and spatial extent indicate that the redundancy of B. franklini has declined
significantly since the late 1990’s.
5.0 Analysis of Future Condition
Due to the lack of observations of the species since 2006, we did not project anticipated future
states of resiliency, redundancy or representation. Numerous survey efforts for invertebrate
pollinators have occurred since 2006 in high quality habitat where Bombus franklini have been
historically observed. During these efforts by Xerces Society, USFS, BLM, FWS, classes at
Southern Oregon University and many private individuals, several species of Bombus have
consistently been observed, but B. franklini has never been found. Although the failure to detect
a species during surveys is not equivalent to a conclusive demonstration of its absence and may
simply reflect the very low detection probability for rare species, the certain losses in both the
number of populations and their spatial extent render B. franklini vulnerable to extinction even
without further external stressors acting upon the species.
Several conservation measures (as described in section 3.3) could be applied to important
Bombus habitats within the historic range of B. franklini, which would be beneficial to other
Bombus species (notably B. occidentalis) and any existing but unknown populations of B.
franklini. These include but are not limited to reductions in herbicide and pesticide applications
and restrictions on the importation and use of commercially produced bees. Expanded and
standardized surveys for B. franklini, B. occidentalis, and other special-status invertebrates
would improve knowledge of species abundance, distribution, and habitat conditions.
43
6.0 Literature Cited
Alaux, C., J. Brunet, C. Dussaubat, F. Mondet, S. Tchamitchan, M. Cousin, J. Brillard, A. Baldy,
L. P. Belzunces, and Y. Le Conte. 2010. Interactions between Nosema microspores and a
neonicotinoid weaken honey bees (Apis mellifera). Environmental Microbiology. 12(3):
774-782.
Alghamdi, A., L. Dalton, A. Phillis, E. Rosato, and E. B. Mallon. 2008. Immune response
impairs learning in free-flying bumble-bees. Biol. Lett. 4, 479–481.
Arena, M. and F. Sgolastra. 2014. A meta-analysis comparing the sensitivity of bees to
pesticides. Ecotoxicology 23:324–334.
Associated Press. 2007. Plight of the Bumblebee, Grants Pass, OR. Accessed on May 17, 2010
online: http://www.cbsnews.com/stories/2007/10/08/tech/main3341254.shtml.
Aufauvre, J., D. G. Biron, C. Vidau, R. Fontbonne, M. Roudel, M. Diogon, B. Viguès, L. P.
Belzunces, F. Delbac and N. Blot. 2012. Parasite-insecticide interactions: A case study of
Nosema ceranae and fipronil synergy on honey bee. Scientific Reports. 2 (326) 1-7.
Bale, J.S., G. J. Masters, I. D. Hodkinson, C. Awmack, T. M. Bezemer, and V. K. Brown. 2002.
Herbivory in global climate change research: direct effects of rising temperature on insect
herbivores. Global Change Biology 8: 1–16.
Baron, G. L, N. E. Raine, and M. J. F. Brown. 2014. Impact of chronic exposure to a pyrethroid
pesticide on bumble bees and interactions with a trypanosome parasite. Journal of
Applied Ecology. 51: 460–469.
Bartomeus, I., J. S. Ascher, D. Wagner, B. N. Danforth, S. Colla, and S. Kornbluth. (2011).
Climate-associated phenological advances in bee pollinators and bee-pollinated plants.
Proc. Natl. Acad. Sci. USA, 108, 20645–20649.
Bhattacharya, M., R. B. Primack, and J. Gerwein. 2003. Are roads and railroads barriers to
bumblebee movement in a temperate suburban conservation area? Biological
Conservation 109: 37-45.
Boggs, C. and D. Inouye, David. 2012. A single climate driver has direct and indirect effects on
insect population dynamics. Ecology letters. 15. 502-8. 10.1111/j.1461-0248.
Brooks, M. 1999. Bumblebee Community Structure in the Marble Mountain Wilderness: Species
Diversity and Foraging Preferences. M.S. thesis, Humboldt State University, Arcata. 43
pp.
Brown, M. J. F., R. Loosli, and P. Schmid-Hempel. 2000. Condition-dependent expression of
virulence in a trypanosome infecting bumble bees. Oikos 91:421-427.

44
Brown, M. J. F., R. Schmid-Hempel, and P. Schmid-Hempel. 2003. Strong context-dependent
virulence in a host–parasite system: reconciling genetic evidence with theory. J. Anim.
Ecol. 72: 994–1002.
Bureau of Land Management. 2015. SW Oregon Integrated Western Bumble Bee Survey
Project- 2015. Summary Report of Findings. Unpublished report prepared for the U.S.
Forest Service and Bureau of Land Management. 15 pp.
California Department of Fish and Wildlife. 2017. California Terrestrial and Vernal Pool
Invertebrates of Conservation Priority. California Department of Fish and Wildlife. 17
pp.
California Department of Fish and Wildlife. 2018. Threatened and Endangered
Invertebrates. California Department of Fish and Wildlife. Accessed online on May 9,
2018 at http://www.dfg.ca.gov/wildlife/nongame/t_e_spp/invertebrates.html.
California Department of Food and Agriculture. 2017. Last modified Plant Health and Pest
Protection Services. https://www.cdfa.ca.gov/plant/pollinators/index.html. Accessed
January 4, 2017.
Cameron, S. A., H. M. Hines, and P. H. Williams. 2007. A comprehensive phylogeny of the
bumble bees (Bombus). Biological Journal of the Linnean Society 91: 161-188.
Cameron, S., S. Jepsen, E. Spevak, J. Strange, M. Vaughan, J. Engler, and O. Byers (eds). 2011a.
North American Bumble Bee Species Conservation Planning Workshop Final Report.
International Union for Conservation of Nature Conservation Breeding Specialist Group:
Apple Valley, MN. Available online at:
http://www.cbsg.org/cbsg/workshopreports/26/bumble_bee_conservation_2010.pdf
Cameron, S. A., J. D. Lozier, J. P. Strange, J. B. Koch; N. Cordes, L. F. Solter, and T. L.
Griswold. 2011b. Patterns of widespread decline in North American bumble bees.
Proceedings of the National Academy of Sciences 108 (2):662-667.
Cameron, S. A., H. C. Lim, J. D. Lozier, M. A. Duennes, and R. Thorp. 2016. Test of the
invasive pathogen hypothesis of bumble bee decline in North America. Proceedings of
the National Academy of Sciences 113 (16): 4386–4391.
Cane, J. H., and J. A. Payne. 1993. Regional, annual, and seasonal variation in pollinator guilds:
intrinsic traits of bees (Hymenoptera: Apoidea) underlie their patterns of abundance at
Vaccinium ashei (Ericaceae). Ann. Entomol. Soc. Am. 86: 577Ð588.
Carvell, C. 2002. Habitat use and conservation of bumblebees (Bombus spp.) under different
grassland management regimes. Biological Conservation 103: 33-49.

45
Carvell, C., D. B. Roy, S. M. Smart, R. F. Pywell, C. D. Preston, and D. Goulson. 2006. Declines
in forage availability for bumblebees at a national scale. Biological Conservation 132:
481-489.
Chauzat, M. P., J. P. Faucon, A. C. Martel, J. Lachaize, N. Cougoule, and M. Aubert. 2006. A
survey of pesticide residues in pollen loads collected by honey bees in France, J. Econ.
Entomol. 99, 253–262.
City of Portland. 2015. Prohibition on Use and Purchase of Neonicotinoid Pesticides by City of
Portland. Binding City Policy- PRK-3.10.
Code, B. H. and S. L. Haney. 2006. Final Report. Franklin’s Bumble Bee Inventory in the
Southern Cascades of Oregon. Unpublished report prepared for the Bureau of Land
Management, Medford, Oregon. 8 pp.
Colla, S. R. and L. Packer. 2008. Evidence for decline in eastern North America bumble bees
(Hymenoptera: Apidae), with special focus on Bombus affinis Cresson. Biodiversity
Conservation 17:1379-1391.
Colla, S. R., M. C. Otterstatter, R.J. Gegear, and J.D. Thomson. 2006. Plight of the bumble bee:
Pathogen spillover from commercial to wild populations. Biological Conservation
129:461-467.
Colyer, S. M. 2016. 2016 Western Bumble Bee Surveys: Rogue River – Siskiyou National
Forest. Unpublished report prepared for the USDA Forest Service. 8 pp.
Coors, A. and L. De Meester. 2008. Synergistic, antagonistic and additive effects of multiple
stressors: Predation threat, parasitism and pesticide exposure in Daphnia magna. J. Appl.
Ecol. 45, 1820–1828.
Corbet, S. A., I. H. Williams, and J. L. Osborne. 1991. Bees and the pollination of crops and
wild flowers in the European Community. Bee World 72:47–59
Cordes, N. J. D. Lozier, W. Huang, L. F. Solter, and J. P. Strange. 2011. Interspecific geographic
distribution and variation of the pathogens Nosema bombi and Crithidia species in United
States bumble bee populations. Journal of Invertebrate Pathology 109 (2): 209-216.
Darvill, B., J. S. Ellis, G. C. Lye, and D. Goulson. 2006. Population structure and inbreeding in a
rare and declining bumble bee, Bombus muscorum (Hymenoptera: Apidae). Molecular
Ecology 15:601-611.
Gloria Degrandi-Hoffman, G.,Y. Chen, E. Watkins Dejong, M. L. Chambers, and G. Hidalgo.
2015. Effects of Oral Exposure to Fungicides on Honey Bee Nutrition and Virus
Levels, Journal of Economic Entomology, Volume 108, Issue 6. p. 2518–2528.

46
Diekotter, T., K. Walther-Hellwig, M. Conradi, M. Suter, and R. Frankl. 2006. Effects of
landscape elements on the distribution of the rare bumblebee species Bombus muscorum
in an agricultural landscape. Biodiversity and Conservation 15: 57-68.
Di Priscoa, G., V. Cavaliere, D. Annoscia, P. Varricchio, E. Caprioa, F. Nazzic, G. Gargiulo, and
F. Pennacchioa. 2013. Neonicotinoid clothianidin adversely affects insect immunity and
promotes replication of a viral pathogen in honey bees. PNAS. 110 (46): 18466-18471.
Douglas, M., and J. F. Tooker. 2015. Large-scale deployment of seed treatments has driven rapid
increase in use of neonicotinoid insecticides and preemptive pest management in U.S.
field crops. Environmental Science and Technology 49:5088-5097.
Dramstad, W. E. 1996. Do bumble bees (Hymenoptera: Apidae) really forage close to their
nests? Journal of Insect Behavior. 9:163-182.
Duchateau, M. J. and H. H. W. Velthuis. 1988. Development and reproductive strategies
in Bombus terrestris colonies. Behaviour 107:186-207.
Durrer, S. and P. Schmid-Hempel. 1994. Shared use of flowers leads to horizontal pathogen
transmission. Proceedings of the Royal Society of London Series Bulletin 258: 299-302.
Ellis, J. S., M. E. Knight, B. Darvill, and D. Goulson. 2006. Extremely low effective population
sizes, genetic structuring and reduced genetic diversity in a threatened bumblebee
species, Bombus sylvarum (Hymenoptera: Apidae). Molecular Ecology 15: 4375-4386.
Elston, C., H. Thompson, K. Walters. 2013. Sub-lethal effects of thiamethoxam, a neonicotinoid
pesticide, and propiconazole, a DMI fungicide, on colony initiation in bumble bee
(Bombus terrestris) micro-colonies. Apidologie DOI: 10.1007/s13592-013-0206-9
Ercan, N. and A. Onus. 2003. The effects of bumblebees (Bombus terrestris L.) on fruit quality
and yield of pepper (Capsicum annuum L.) grown in an unheated greenhouse. Israel
Journal of Plant Sciences. 51. p. 275-283.
Evans, E. C. 2001. Competition between European honey bees and native bumblebees: resource
overlap and impact on reproductive success. Master’s Thesis, University of Minnesota.
Fauser-Misslin, A., B. M. Sadd, P. Neumann, and C. Sandrock. 2014. Influence of combined
pesticide and parasite exposure on bumble bee colony traits in the laboratory. Journal of
Applied Ecology 51:450-459.
Feltham, H., K. Park, and D. Goulson. 2014. Field realistic doses of pesticide imidacloprid
reduce bumble bee pollen foraging efficiency. Ecotoxicology 23:317-323.
Fitzpatrick, U., T. E. Murray, R. J. Paxton, J. Breen, D. Cotton, V. Santorum, and M. J. F.
Brown. 2007. Rarity and decline in bumble bees: a test of causes and correlates in the
Irish fauna. Biological Conservation 136:185-194.
47
Flanders, R. V., W. F. Wehling, and A. L. Craghead. 2003. Laws and Regulations on the Import,
Movement, and Release of Bees in the United States. Entomological Society of America,
Madison, Wisconsin: 99-111.
Forister, M. L., A. C. McCall, N. J. Sanders, J. A. Fordyce, J. H. Thorne, J. O’Brien, D. P.
Waetjen, and A. M. Shapiro. 2010. Compounded effects of climate change and habitat
alteration shift patterns of butterfly diversity. PNAS 107(5): 2088-2092.
Frankie, G. W., R. W. Thorp, M. Schindler, J. Hernandez, B. Ertter, and M. Rizzardi. 2005.
Ecological patterns of bees and their host ornamental flowers in two northern California
cities. Journal of the Kansas Entomological Society. 78: 227-246.
Frison, T. H. 1921. New distribution records for North American Bremidae, with the description
of a new species (Hym.) Entomological News 32: 144-148.
Frison, T. H. 1922. Systematic and biological notes on bumblebees (Bremidae; Hymenoptera).
Transactions of the American Entomological Society 48: 307-326.
Fürst, M. A., McMahon, D. P., Osborne, J. L., Paxton, R. J., & Brown, M. J. F. (2014). Disease
associations between honeybees and bumblebees as a threat to wild pollinators. Nature,
506(7488), 364
Gegear, R. J., M. C. Otterstatter, and J. D. Thomson. 2005. Does parasitic infection impair the
ability of bumblebees to learn flower-handling techniques? Animal Behaviour 70: 209-
215.
Gegear, R. J., M. C. Otterstatter, and J. D. Thomson. 2006. Bumble-bee foragers infected by a
gut parasite have an impaired ability to utilize floral information. Proceedings of the
Royal Society B. 273: 1073-1078.
Gels, J., D. W. Held, and D. A. Potter. 2002. Hazards of insecticides to the bumble bees Bombus
impatiens (Hymenoptera: Apidae) foraging on flowering white clover in turf. Journal of
Economic Entomology 95: 722-728.
Genersch, E., C. Yue, I. Fries, and J. R. de Miranda. 2006. Detection of Deformed wing virus, a
honey bee viral pathogen, in bumble bees (Bombus terrestris and Bombus pascuorum)
with wing deformities. Journal of Invertebrate Pathology 91: 61-63.
Gibson, C., V. Brown, L. Losito, and G. McGavin. 1992. The response of invertebrate
assemblies to grazing. Ecography 15: 166-176.
Gill, R. J. and N. E. Raine. 2014. Chronic impairment of bumble bee natural foraging behaviour
induced by sublethal pesticide exposure. Functional Ecology. doi: 10.1111/1365-
2435.12292.

48
Gill, R. J., O. Ramos-Rodriguez, N. E. Raine. 2012. Combined pesticide exposure severely
affects individual- and colony-level traits in bees. Nature 491, 105–108.
doi:10.1038/nature11585; pmid: 23086150.
Goka, K., K. Okabe, M. Yoneda, and S. Niwa. 2001. Bumblebee commerce will cause
worldwide migration of parasitic mites. Molecular Ecology 10: 2095-2099.
Goodell, K. and J. D. Thomson. 1997. Comparisons of pollen removal and deposition by honey
bees and bumble bees visiting apple. Acta Horticultura 437: 103-107.
Goodwin, R. M. and H. M. McBrydie. 2000. Effect of surfactants on honey bee survival. N Z Pl
Protect 53: 230–234.
Goulson, D. 2013. An overview of the environmental risks posed by neonicotinoid insecticides.
Journal of Applied Ecology 50:977–987.
Goulson, D. 2010. Bumblebees: behavior, ecology, and conservation. Oxford University Press,
Oxford, UK. 317 pp.
Goulson, D. and K. R. Sparrow. 2009. Evidence for competition between honeybees and
bumblebees; effects on bumblebee worker size. Journal of Insect Conservation 13: 177-
181.
Goulson, D., G. C. Lye, and B. Darvill. 2008. Decline and conservation of bumble bees. Annual
Review of Entomology 53:191-208.
Goulson, D., E. Nicholls, C. Bouias, E.L. Rotheray. 2015. Bee declines driven by combined
stress from parasites, pesticides, and lack of flowers. Science 347 (6229): 1255957-9.
Grixti, J., L.T. Wonga, S.A. Cameron, and C. Favreta. 2009. Decline of bumble bees (Bombus)
in the North American Midwest. Biological Conservation 142: 75-84.
Hanski, I. 1999. Metapopulation ecology. Oxford University Press, New York.
Hatfield, R. G. and G. LeBuhn. 2007. Patch and landscape factors shape community assemblage
of bumble bees, Bombus spp. (Hymenoptera: Apidae), in montane meadows. Biological
Conservation 139: 150-158.
Hendry, A. P., M. T. Kinnison, M. Heino, T. Day, T. B. Smith, G. Fitt, C. T. Bergstrom, J.
Oakeshott, P. S Jorgensen, M. P. Zalucki, G.Gilchrist, S. Southerton, A. Sih, S. Strauss,
R .F Denison, and S. P. Carroll. 2011. Evolutionary principals and their practical
application. Evolutionary Applications 4:159-183.
Herrmann, F., C. Westphal, R. F. A. Moritz, and I. Steffan-Dewenter. 2007. Genetic diversity
and mass resources promote colony size and forager densities of a social bee (Bombus
pascuorum) in agricultural landscapes. Molecular Ecology: 1167-1178.
49
Hickling, R., D. B. Roy, J. K. Hill, R. Fox, and C. D. Thomas. 2006. The distributions of a wide
range of taxonomic groups are expanding polewards. Global Change Biology. 12:450-
455.
Hines, H. M. and S. D. Hendrix. 2005. Bumble bee (Hymenoptera: Apidae) diversity and
abundance in tallgrass prairie patches: Effects of local and landscape flora resources.
Environmental Entomology 34(6): 1477-1484.
Hobbs, G. A. 1968. Ecology of species of Bombus (Hymenoptera: Apidae) in southern Alberta.
VII. Subgenus Bombus. Canadian Entomologist 100: 156-164.
Holm, S. N. 1966a. The utilization and management of bumble bees for red clover and alfalfa
seed production. A. Rev. Ent. 11: 155 – 182.
Holm, S. N. 1966b. Problems of the domestication of bumble bees. Bee World 47, 179-186
Høye, T. T., E. Post, N. M. Schmidt, K. Trøjelsgaard, and M. C. Forchhammer. 2013. Shorter
flowering seasons and declining abundance of flower visitors in a warmer Arctic. Nat.
Clim. Change. 3: 759–763.
Huntzinger, M. 2003. Effects of fire management practices on butterfly diversity in the forested
western United States. Biological Conservation 113: 1-12.
Ings, T. C., N. L. Ward, and L. Chittka. 2006. Can commercially imported bumble bees out-
compete their native conspecifics? Journal of Applied Ecology 43: 940-948.
Integrated Taxonomic Information System. 2017. Last updated November 8
th
, 2017.
https://www.itis.gov/. Accessed September 2017.
International Union for Conservation of Nature. 2009. Red List of Threatened Species. Available
online: http://www.iucnredlist.org/details/135295 (accessed 26 February 2009).
Interagency Special Status/Sensitive Species Program. 2018. ISSSP Species Index. U. S. Forest
Service and Bureau of Land Management. Accessed online on May 9, 2018 at
https://www.fs.fed.us/r6/sfpnw/issssp/species-index/fauna-invertebrates.shtml.
Intergovernmental Panel on Climate Change. 2013: Climate Change 2013: The Physical Science
Basis. Contribution of Working Group I to the Fifth Assessment Report of the
Intergovernmental Panel on Climate Change [Stocker, T.F., D. Qin, G.-K. Plattner, M.
Tignor, S.K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex and P.M. Midgley (eds.)].
Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA,
1535 p
50
Iwasa, T., N. Motoyama, J. T. Ambrose, and R. M. M. Roe. 2004. Mechanism for the differential
toxicity of neonicotinoid insecticides in the honey bee, Apis mellifera. Crop Prot. 23,
371–378.
Jilian, L., W. Jie, P. Wenjun, A. Jiandong, G. Zhanbao, and T. Yuemin. 2005. Nosema bombi, a
microsporidian pathogen of the bumble bee Bombus lucorum. Journal of Apicultural
Science 49: 53-57.
Johansen, C. A. 1977. Pesticides and pollinators. Annual Review of Entomology 22: 177-192.
Johansen, C. A. and D. F. Mayer. 1990. Pollinator Protection: a Bee and Pesticide Handbook.
Wicwas Press, Cheshire, Connecticut.
Johnson, M. D. and C. M. Horn. 2008. Effects of rotational grazing on rodents and raptors in a
coastal grassland. Western North American Naturalist 68: 444-452.
Kearns, C. A., D. W. Inouye, and N. Waser. 1998. Endangered mutualisms: the conservation of
plant–pollinator interactions. Annual Review of Ecology and Systematics 29: 83-112.
Kearns, C. A. and D. W. Inouye. 1997. Pollinators, flowering plants, and conservation biology.
BioScience 47: 297-307.
Kerr, J. T., Kerr, S. M. Roberts, P. Rasmont, O. Schweiger, S. R. Colla, L. L. Richardson, D. L.
Wagner, L. F. Gall, D. S. Sikes, and A. Pantoja. 2015. Climate change impacts on
bumble bees converge across continents. Science 349(6244): 177-180.
Kevan, P. G. 1975. Forest application of the insecticide Fenitrothion and its effect on wild bee
pollinators (Hymenoptera: Apoidea) of lowbush blueberries (Vaccinium spp.) in southern
New Brunswick, Canada. Biological Conservation 7: 301-309.
Kevan, P. G. 1999. Pollinators as bioindicators of the state of the environment: species, activity
and diversity. Agriculture Ecosystems and Environment 74: 373-393.
Kissinger, C. N., S. A. Cameron, R. W. Thorp, B. White, and L. F. Solter. 2011. Survey of
bumble bee (Bombus) pathogens and parasites in Illinois and selected areas of northern
California and southern Oregon. Journal of Invertebrate Pathology 107:220-224.
Knight, M. E., A. P. Martin, S. Bishop, J. L. Osborne, R. J. Hale, A. Sanderson, and D. Goulson.
2005. An interspecific comparison of foraging range and nest density of four bumble bee
(Bombus) species. Molecular Ecology 14:1811–1820.
Koch, J. B., J. P. Strange, and P. Williams. 2012. Bumble Bees of the Western United States.
USDA-Forest Service. (http:// www.pollinator.org/PDFs/
BumbleBee.GuideWestern.FINAL. pdf) (accessed 10 April 2015).

51
Kosior, A., W. Celary, P. Olejniczak, J. Fijat, W. Krol, W. Solarz, and P. Plonka. 2007. The
decline of the bumble bees and cuckoo bees (Hymenoptera: Apidae: Bombini) of western
and central Europe. Oryx 41: 79-88.
Kosior, G., A. Samecka-Cymerman, K. Kolon, and A.J. Kempers. 2010. Bioindication capacity
of metal pollution of native and transplanted Pleurozium schreberi under various levels of
pollution. Chemosphere. 2010 Sep; 81(3):321-6.
Krupke, C. H., G. J. Hunt, B. D. Eitzer, G. Andino, and K. Given. 2012. Multiple routes of
pesticide exposure for honey bees living near agricultural fields. PLoS
One. 2012;7:e29268.
Kruess, A. and T. Tscharntke. 2002a. Contrasting responses of plant and insect diversity to
variation in grazing intensity. Biological Conservation 106: 293-302.
Kruess, A, and T. Tscharntke. 2002b. Grazing intensity and the diversity of grasshoppers,
butterflies, and trap-nesting bees and wasps. Conservation Biology 16: 1570-80.
Kudo, G. and T. Y. Ida. 201. Early onset of spring increases the mismatch between plants and
pollinators. Ecology. 94. 2311-20.
LaLande, J. M. 1995. An Environmental History of the Little Applegate River
Watershed, Jackson County, Oregon. U.S. Dept. of Agriculture, Forest Service, Rogue
River National Forest. Medford, Oregon.
Laycock, I., K. M. Lenthall, A. T. Barratt, and J. E. Cresswell. 2012. Effects of imidacloprid, a
neonicotinoid pesticide, on reproduction in worker bumble bee (Bombus terrestris).
Ecotoxicology 21, 1937–1945.
Le Féon, V., A. Schermann-Legionnet, Y. Delettre, S. Aviron, R. Billeter, R. Bugter, F.
Hendrick, and F. Burel. 2010. Intensification of agriculture, landscape composition and
wild bee communities: A large scale study in four European countries. Agriculture,
Ecosystems & Environment 137: 143-150.
Loken, A. 1958. Pollination studies in apple orchards of western Norway. In 10th Internatl.
Cong. Ent. Proc. 4: 961-965. Aug. 17-25, 1956, Montreal.
Lundin, O., M. Rundlöf, H. G. Smith, I. Fries, and R. Bommarco. 2015. Neonicotinoid
insecticides and their impacts on bees: A systematic review of research approaches and
identification of knowledge gaps. PLoS ONE 10:1-20.
MacFarlane, R. P., K. D. Patten, L. A. Royce, B. K. W. Wyatt, and D. F. Mayer. 1994.
Management Potential of Sixteen North American Bumble Bee Species. Melanderia
50:1-12.
52
Macfarlane, R. P., J. J. Lipa, and H. J. Liu. 1995. Bumble bee pathogens and internal enemies.
Bee World 76: 130-148.
Macfarlane, R. P. and K.D. Patten. 1997. Food sources in the management of bumble bee
populations around cranberry marshes. In Proceedings of the 7th International
Symposium on Pollination. K. W. Richards (ed.) Acta Horticulturae: 239-244.
MacKenzie, K.E. and A.L. Averill. 1995. Bee (Hymenoptera: Apoidea) diversity and abundance
on cranberry in southeastern Massachusetts. Ecology and Population Biology, 88, 334-
341.
Malfi, R.L. and T. Roulston. 2014. Patterns of parasite infection in bumble bees (Bombus spp.)
of Northern Virginia. Ecological Entomology 39:17–29.
Manley, R., M. Boots, and L. Wilfert. 2015. Emerging viral disease risk to pollinating insects:
ecological, evolutionary and anthropogenic factors. Journal of Applied Ecology doi:
10.1111/1365-2664.12385.
Matsumura, C., M. Nakajima, J. Yokoyama, and I. Washitani. 2004. High reproductive ability of
an alien bumblebee invader, Bombus terrestris L., in the Hidaka region of southern
Hokkaido, Japan. Japanese Journal of Conservation Ecology 9: 93-101.
Mayer, D. F., K. D. Patten, and R. P. MacFarlane. 2004. Pear Pollination with Managed Bumble
Bee (Hymenoptera:Apidae) Colonies. Melanderia 50:20-23.
Mayer, D. F. and J. D. Lunden. 1997. A Comparison of Commercially Managed Bumblebees
and Honey Bees (Hymenoptera:Apidae) for Pollination of Pears. Proceedings of the
International Symposium on Pollination, 283-287.
Mayer, D. F. and J. D. Lunden. 2001. Honey Bee Management and Wild Bees for Pollination of
Hybrid Onion Seed. Proceedings from the 8
th
Pollination Symposium, 275-278.
McFrederick, Q. S. and G. LeBuhn. 2006. Are urban parks refuges for bumble bees Bombus spp.
(Hymenoptera: Apidae)? Biological Conservation 129: 372-382.
Meeus, I., M.J.F. Brown, D.C. De Graaf, and G. Smagghe. 2011. Effects of Invasive Parasites on
Bumble Bee Declines. Conservation Biology 25(4):662-671.
Memmott, J., P. G. Craze, N. M. Waser, and M. V. Price. 2007. Global warming and the
disruption of plant-pollinator interactions. Ecology Letters 10:710-717.
Milliron, H. E. 1971. A Monograph of the Western Hemisphere Bumblebees (Hymenoptera:
Apidae: Bombus. The Genera Bombus and Megabombus Subgenus Bombias). The
Entomological Society of Canada, Ottawa- Memoir No. 82. 44 pp.

53
Mommaerts V, G. Sterk, and G. Smagghe. 2006. Hazards and uptake of chitin synthesis
inhibitors in bumble bees Bombus terrestris. Pest Management Science 62:752-758.
Mommaerts, V., S. Reynders, J. Boulet, L. Besard, G. Sterk, and G. Smagghe. 2010. Risk
assessment for side-effects of neonicotinoids against bumble bees with and without
impairing foraging behavior. Ecotoxicology. 19:207-215.
Mommaerts V., P. Kurt, and G. Smagghe. 2011. Bombus terrestris as pollinator-and-vector to
suppress Botrytis cinerea in greenhouse strawberry. Pest Manag Sci 67:1069–1075.
Morandin, L. A., M. L. Winston, M. T. Franklin, and V. A. Abbott. 2005. Lethal and sublethal
effects of spinosad on bumble bees (Bombus impatiens Cresson). Pest Management
Science 61: 619-626.
Moret, Y. and P. Schmid-Hempel. 2000. Survival for immunity: the price of immune system
activation for bumblebee workers. Science.290, 1166–1168.
Morris, M. G. 1967. Differences between invertebrate faunas of grazed and ungrazed chalk
grassland. I. Responses of some phytophagous insects to cessation of grazing. Journal of
Applied Ecology 4: 459-474.
Mullin, C. A., M. Frazier, J. L. Frazier, S. Ashcraft, R. Simonds, D. VanEngelsdorp, and J. S.
Pettis. 2010. High levels of miticides and agrochemicals in North American apiaries:
implications for honey bee health. PLoS ONE. 2010;5:e9754.
Mullin, C. A., J. Chen, J. D. Fine, M. T. Frazier, and J. L. Frazier. The formulation makes the
honey bee poison, Pesticide. Biochemistry and Physiology (2015), doi:
10.1016/j.pestbp.2014.12.026. 2. C.A
Murray, T. E., M. F. Coffey, E. Kehoe, and F. G. Horgan. 2013. Pathogen prevalence in
commercially reared bumble bees and evidence of spillover in conspecific populations.
Biological Conservation 159:269-276.
National Research Council. 2007. Status of Pollinators in North America. The National
Academies Press, Washington, DC.
Niwa, S., H. Iwano, S. Asada, M. Matsuura, and K. Goka. 2004. A Microsporidian Pathogen
Isolated from a Colony of the European Bumblebee, Bombus terrestris, and Infectivity on
Japanese Bumble bee. Japanese Journal of Applied Entomology and Zoology, 48:60-64.
Öckinger, E. and H. G. Smith. 2007. Semi-natural grasslands as population sources for
pollinating insects in agricultural landscapes. Journal of Applied Ecology 44: 50-59.
Ogilvie, D., L. Foley, A. Nimegeer, J. Olsen, R. Mitchell, H. Thomson, F. Crawford, R. Prins, S.
Hilton, A. Jones, D. Humphreys, S. Sahlqvist, and N. Mutrie. 2017. Health impacts of the

54
M74 urban motorway extension: a mixed-method natural experimental study. Public
Health Res., 5 (2017), p. 3.
Oregon Department of Agriculture. 2017. Oregon Approved Invertebrate List. Accessed online
March 26
th
2018 at
http://www.oregon.gov/ODA/shared/Documents/Publications/IPPM/OregonApprovedInv
ertebrateList.pdf
Oregon Department of Fish and Wildlife. 2018. Threatened, Endangered, and Candidate Fish and
Wildlife Species. Oregon Department of Fish and Wildlife. Accessed online on May 9,
2018 at
https://www.dfw.state.or.us/wildlife/diversity/species/threatened_endangered_candidate_
list.asp
Osborne, J.L., S.J. Clark, R.J. Morris, I.H. Williams, J.R. Riley, A.D. Smith, D.R. Reynolds, and
A.S. Edwards. 1999. A landscape-scale study of bumble bee foraging range and
constancy, using harmonic radar. Journal of Applied Ecology 36:519-533.
Otterstatter, M. C., R. J. Gegear, S. R. Colla, and J. D. Thomson. 2005. Effects of parasitic mites
and protozoa on the flower constancy and foraging rate of bumble bees. Behavioral
Ecology and Sociobiology 58: 383–389.
Otterstatter, M. C., and J. D. Thomson. 2008. Does pathogen spillover from commercially reared
bumble bees threaten wild pollinators? PLoS ONE 3:
e2771.doi:10.1371/journal.pone.0002771. Available online:
http://www.plosone.org/doi/pone.0002771 (accessed 5 March 2009).
Otterstatter, M. C. and T. L. Whidden. 2004. Patterns of parasitism by tracheal mites
(Locustacarus buchneri) in natural bumble bee populations. Apidologie 35: 351-357.
Otti, O. and P. Schmid-Hempel. 2007. Nosema bombi: A pollinator parasite with detrimental
fitness effects. Journal of Invertebrate Pathology 96:118–124.
Otti, O. and P. Schmid-Hempel. 2008. A field experiment on the effect of Nosema bombi in
colonies of the bumblebee Bombus terrestris. Ecological Entomology 33: 577–582.
Packer, L. and R. Owen. 2001. Population genetic aspects of pollinator decline. Conservation
Ecology 5: Article 4.
Panzer, R. 2002. Compatibility of prescribed burning with the conservation of insects in small,
isolated prairie reserves. Conservation Biology 16: 1296-1307.
Pettis, J. S., D. vanEngelsdorp, J. Johnson, and G. Dively. 2012. Pesticide exposure in honey
bees results in increased levels of the gut pathogen Nosema. Naturwissenschaften 99,
153–158.

55
Pettis, J. S., E. M. Lichtenberg, M. Andree, J. Stitzinger, R. Rose, and D. vanEngelsdorp. 2013.
Crop Pollination Exposes Honey Bees to Pesticides Which Alters Their Susceptibility to
the Gut Pathogen Nosema ceranae. PLoS ONE 8(7): e70182.
Piiroinen, S. and D. Goulson. 2016. Chronic neonicotinoid pesticide exposure and parasite stress
differentially affects learning in honeybees and bumblebees. Proc. R. Soc. B. 283:
20160246
Piling, E. D. and P. C. Jepsen. 1993. Synergism between EBI fungicides and a pyrethroid
insecticide in the honey bee (Apis mellifera). Pesticide Science 39(4) 293-297.
Plath, O. E. 1927. Notes on the Nesting Habits of Some of the Less Common New England
Bumble-Bees. Psyche 34:122-128.
Ploquin, E. F., J. M. Herrera, and J. R. Obeso. 2013. Bumblebee community homogenization
after uphill shifts in montane areas of northern Spain. Oecologia 173: 1649-1660.
Plowright, R. C. and W. P. Stephen. 1980. The taxonomic status of Bombus franklini
(Hymenoptera: Apidae). Canadian Entomologist 112: 475-479.
Plowright, R. C., B. A. Pendrel, and I. A. McLaren. 1978. The Impact of Aerial Fenitrothion
Spraying upon the Population Biology of Bumble Bees (Bombus Latr: Hym) in South-
Western New Brunswick. The Canadian Entomologist110: 1145-1156
Plowright, R. C., J. D. Thomson, and G. R. Thaler. 1980. Pollen removal in Cypripedium acaule
(Orchidae) in relation to aerial fenitrithion spraying in New Brunswick. Canadian
Entomologist 112: 765-769.
Pisa, L. W., V. Amaral-Rogers, L. P. Belzunces, J. M. Bonmatin, C. A. Downs, D. Goulson,
D. P. Kreutzweiser, C. Krupke, M. Liess, M. McField, C. A. Morrissey, D. A. Noome,
J. Settele, N. Simon-Delso, J. D. Stark, J. P. Van der Sluijs, H. Van Dyck, and
M. Wiemers. 2015. Effects of neonicotinoids and fipronil on non-target invertebrates.
Environ. Sci. Pollut. Res. Int. 22, 68–102.
Pool, E. 2014. Final Report. Meadow Invertebrate Detections in Southern Oregon. Unpublished
report prepared for the Bureau of Land Management, Medford, Oregon. 11 pp.
Potts, S. G., J. C. Biesmeijer, C. Kremen, P. Neumann, O. Schweiger, and W. E. Kunin. 2010.
Global pollinator declines: Trends, impacts and drivers. Trends in Ecological Evolution
25:345–353.
Powell, M. 2017. Aerial Spray Ban: Lincoln County passes a ballot measure banning the aerial
spraying of pesticides. Eugene Weekly, May 15, 2017. Accessed online on May 9, 2018
at https://www.eugeneweekly.com/2017/06/15/aerial-spray-ban/.

56
Pyke, G. H., J. D. Thomson, D. W. Inouye, and T. J. Miller. 2016. Effects of climate change on
phenologies and distributions of bumble bees and the plants they visit. Ecosphere
7(3):e01267. 10.1002/ecs2.1267
Rao, S. and J. P. Strange. 2012. Bumble Bee (Hymenoptera: Apidae) Foraging Distance and
Colony Density Associated With a Late-Season Mass Flowering Crop. Environmental
Entomology, 41(4):905-915.
Ratti, C. M. and S. Colla. 2010. Discussion of the presence of an eastern bumble bee
species (Bombus impatiens Cresson) in western Canada. Pan-Pacific Entomologist.
Richards, K. W. and T. W. Myers. 1997. Commercially Managed Colonies of Bumblebees for
Pollination of Cicer Milkvetch. Proceedings of the International Symposium on
Pollination, 293-297.
Riddell, C. E. and E. B. Mallon. 2006. Insect psychoneuroimmunology: Immune response
reduces learning in protein starved bumble bees (Bombus terrestris). Brain Behav.
Immun. 20, 135–138.
Roland, J., and S. F. Matter. 2016. Pivotal effect of early-winter extreme temperatures and
snowfall on annual population growth of alpine Parnassius
smintheus butterflies. Ecological Monographs 86:412–428.
Ruchty, A. 2011. Conservation Strategy for Sisyrinchium sarmentosum Suks. ex. Greene. USDA
Forest Service, USDI Bureau of Land Management, Oregon and Washington. 67pp.
Ruiz-Gonzalez, M. X. and M. J. F. Brown. 2006. Honey bee and bumblebee trypanosomatids:
specificity and potential for transmission. Ecological Entomology 31: 616-622.
Rusterholz, H. P. and Baur, B. 2010. Delayed response in a plant–pollinator system to
experimental grassland fragmentation. Oecologia 163: 141-152.
Rutrecht, S. T. and M. J. F. Brown. 2009. Within colony dynamics of Nosema bombi infections:
disease establishment, epidemiology and potential vertical transmission. Apidologie
39:504–514.
Rutrecht. S. T., J. Klee, and M. J. F. Brown. 2007. Horizontal transmission success of Nosema
bombi to its adult bumble bee hosts: effects of dosage, spore source and host age.
Parasitology. 134: 12: 1719-1726.
Sanchez-Bayo, F., and K. Goka. 2014. Pesticide residues and bees – a risk assessment. PLOS
ONE 9 e94482. Doi: 10.1371/journal.pone.00094482; pmid:2478419.
Schmidt, N. M., H. Olsen, and H. Leirs. 2009. Livestock grazing intensity affects abundance of
Common shrews (Sorex araneus) in two meadows in Denmark. BMC Ecology
57
9:2doi:10.1186/1472-6785-9-2. Available online: http://www.biomedcentral.com/1472-
6785/9/2 (accessed 17 May 2010).
Schmuck, R., T. Stadler, and H. W. Schmidt. 2003. Field relevance of a synergistic effect
observed in the laboratory between an EBI fungicide and a chloronicotinyl insecticide in
the honey bee (Apis mellifera L, Hymenoptera) Pest Manag Sci. 59:279–286.
Scholl, A., R. W. Thorp, and E. Obrecht. 1992. The genetic relationship between Bombus
franklini (Frison) and other taxa of the subgenus Bombus s. str. (Hymenoptera: Apidae).
Pan-Pacific Entomologist 68: 46-51.
Scholer, J. and V. Krischik. 2014. Chronic Exposure of Imidacloprid and Clothianidin Reduce
Queen Survival, Foraging, and Nectar Storing in Colonies of Bombus impatiens. PLoS
ONE 9(3): e91573.
Shaffer, M. L., and B. A. Stein. 2000. Safeguarding our precious heritage. Precious heritage: the
status of biodiversity in the United States. Oxford University Press, New York: 301-321.
Shepherd, M., S. L. Buchmann, M. Vaughan, and S. H. Black. 2003. Pollinator Conservation
Handbook. The Xerces Society, Portland, Oregon.
Shipp, J. L., G. H. Whitfield, and A. P. Papadopoulos. 1994. Effectiveness of the bumble bee,
Bombus impatiens Cr. (Hymenoptera: Apidae), as pollinator of greenhouse sweet pepper.
Scientia Horticulturae 57: 29-39.
Shultz, C.B. and E. E. Crone. 1998. Burning Prairie to Restore Butterfly Habitat: A Modeling
Approach to Management Tradeoffs for the Fender’s Blue. Restoration Ecology Vol. 6
No. 3, pp. 244–252.
Sih, A., A. M. Bell, and J. L. Kerby. 2004. Two stressors are far deadlier than one. Trends Ecol.
Evol. 19, 274–276.
Smallidge, P. J. and D. J. Leopold. 1997. Vegetation management for the maintenance and
conservation of butterfly habitats in temperate human-dominated habitats. Landscape and
Urban Planning 38: 259-280.
Solter, L. F., S. A. Cameron, R. W. Thorp, and B. White. 2010. Survey for Nosema bombi: a
Potential Causative Agent for Decline of Bombus franklini and Bombus occidentalis.
Final Report: U.S. Fish & Wildlife Purchase Order #101817M496
Sponsler, D. B., & Johnson, R. M. (2017). Poisoning a Society: A Superorganism Perspective on
Honey Bee Toxicology. Bee World, 94(1), 30-32.
Stephen, W. P. 1957. Bumble bees of western America (Hymenoptera: Apoidea). Oregon State
College Agricultural Experiment Station: Technical Bulletin No. 40. 163pp.

58
Stokstad, E. 2013. Pesticides under fire for risks to pollinators. Science. 2013 May
10;340(6133):674-6.
Stubbs, C. S. Drummond F. A. 2001. Bombus impatiens (Hymenoptera: Apidae): an alternative
to Apis mellifera (Hymenoptera: Apidae) for lowbush blueberry pollination.Journal of
Ecological Entomology 94: 609-616.
Sugden, E. 1985. Pollinators of Astragalus monoensis Barneby (Fabaceae) - New host records -
Potential impact of sheep grazing. Great Basin Naturalist 45: 299-312.
Szabo, N. D., S. R. Colla, D. L. Wagner, L. F. Gall, and J. T. Kerr. 2012. Conservation Letters 5:
232-239.
Thompson, H. M. 2001. Assessing the exposure and toxicity of pesticides to bumblebees
Bombus sp.). Apidologie 32: 305-321.
Thomson, D. 2004. Competitive interactions between the invasive European honey bee and
native bumble bees. Ecology 85: 458-470.
Thomson, D. M. 2006. Detecting the effects of introduced species: a case study of competition
between Apis and Bombus. Oikos 114: 407-418.
Thomson, D. M. 2016. Local bumble bee decline linked to recovery of honey bees, drought
effect on floral resources. Ecology Letters Vol. 19, #10, 1247-1255.
Thompson, H. M., and L.V. Hunt. 1999. Extrapolating from honey bees to bumble bees in
pesticide risk assessment. Ecotoxicology 8:147-166.
Thompson, H. M., S. Wilkins, S. Harkin, S. Milner, and K. F. A. Walters. 2014. Neonicotinoids
and bumble bees (Bombus terrestris): effects on nectar consumption in individual
workers. Wiley Online Library at: wileyonlinelibrary.com DOI 10.1002/ps.3868.
Thorp, R. W. 1970. The type locality of Bombus franklini and notes on putative Arizona records
of other Bombini (Hymenoptera: Apidae). Pan-Pacific Entomologist 46: 177-180.
Thorp, R. W., D. S. Horning, Jr., and L. L. Dunning. 1983. Bumble bees and cuckoo bumble
bees of California. Bulletin of the California Insect Survey 23: 1-79.
Thorp, R. W. 1999. Franklin’s Bumble Bee, Bombus franklini (Frison 1921): a species of special
concern. Unpublished report prepared for the USDA Forest Service, Ashland Ranger
District. 20 pp.
Thorp, R. W. 2001. Franklin’s Bumble Bee, Bombus franklini (Frison 1921): a species of special
concern. Unpublished report prepared for the U.S. Fish and Wildlife Service, Portland
Office. 13 pp.

59
Thorp, R. W. 2003. Bumble bees (Hymenoptea: Apidae): commercial use and environmental
concerns. Pp. 21-40. In: K. Strickler and J. H. Cane (eds.). For nonnative crops, whence
pollinators of the future? Thomas Say Publications in Entomology: Proceedings.
Entomological Society of Amererica, Lanham, MD., 204pp.
Thorp, R. W. 2004. Franklin’s Bumble Bee, Bombus (Bombus) franklini (Frison) (Hymenoptera:
Apidae). Unpublished report prepared for the U.S. Fish and Wildlife Service, Portland
Office. 13 pp.
Thorp, R. W. 2005a. Franklin’s Bumble Bee, Bombus (Bombus) franklini (Frison)
(Hymenoptera: Apidae). Unpublished report prepared for the U.S. Fish and Wildlife
Service, Portland Office. 15 pp.
Thorp, R. W. 2005b. Species Profile: Bombus franklini. In Shepherd, M. D., D. M. Vaughan, and
S. H. Black (Eds). Red List of Pollinator Insects of North America. CD-ROM Version 1
(May 2005). Portland, OR: The Xerces Society for Invertebrate Conservation.
Tyler, E. R., S. Adams, and E. B. Mallon. 2006. An immune response in the bumble bee,
Bombus terrestris leads to increased food consumption. BMC Physiology. 6:1-4.
University of California. 2009. Franklin's bumble bee may be extinct. University of California.
Davis, California. https://phys.org/news/2009-05-franklin-bumble-bee-extinct.html.
Retrieved on 1/12/18
U.S. Environmental Protection Agency. 2018. State and Tribal Managed Pollinator Protection
Plans. Washington, D.C. https://www.epa.gov/pollinator-protection/policy-mitigating-
acute-risk-bees-pesticide-products. Accessed 5/3/2018.
U.S. Environmental Protection Agency. 2017. Policy Mitigating Acute Risk to Bees from
Pesticide Products. Washington, D.C. https://www.epa.gov/pollinator-protection/policy-
mitigating-acute-risk-bees-pesticide-products. Accessed 5/3/2018.
U.S. Fish and Wildlife Service. 2011. 90-Day Finding on a Petition to List the Franklin’s
Bumble Bee as Endangered. Federal Register 76:56381-56391.
U.S. Fish and Wildlife Service. 2014. Memorandum- Use of Agricultural Practices in Wildlife
Management in the National Wildlife Refuge System. U. S. Fish and Wildlife Service,
Washington, D. C. 2pp.
U.S. Fish and Wildlife Service. 2016a. Rusty Patch Bumble Bee (Bombus affinis) Species Status
Assessment. 94 pp.
U.S. Fish and Wildlife Service. 2016b. Survey data, B. franklini and B. occidentalis surveys, July
2016. Unpublished data.

60
U.S. Fish and Wildlife Service. 2017. Survey data, B. franklini and B. occidentalis surveys, July
2016. Unpublished data.
U.S. Geological Survey. 2016. National Pesticide Synthesis Website
http://water.usgs.gov/nawqa/pnsp/usage/maps/index.php. Accessed November 2017),
Vazquez, D. P., and D. Simberloff. 2003. Changes in interaction biodiversity induced by an
introduced ungulate. Ecology Letters 6: 1077-1083.
Velthius, H. H. W. and A. van Doorn. 2006. A century in advances in bumblebee domestication
and the economic and environmental aspects of its commercialization for pollination.
Apidologie 37: 421-451.
Vidau C., M. Diogon, J. Aufauvre, R. Fontbonne, B. Viguès, J. L. Brunet, C. Texier, D. G.
Biron, N. Blot, H. El Alaoui, L. P. Belzunces, and F. Delbac. 2011. Exposure to sublethal
doses of fipronil andthiacloprid highly increases mortality of honey bees previously
infected by Nosema ceranae. PLOS ONE 6, e21550.
Whitehorn, P. R., S. O’Connor, F. L. Wackers, and D. Goulson. 2012. Neonicotinoid pesticide
reduces bumble-bee colony growth and queen production. Science 336, 351–352.
Whittington, R., M. L. Winston, C. Tucker, and A. L. Parachnowitsch. 2004. Plant-species
identity of pollen collected by bumblebees placed in greenhouses for tomato pollination.
Canadian Journal of Plant Science 84: 599-602.
Williams, P. H. 1986. Environmental change and the distribution of British bumble bees
(Bombus Latr.). Bee World 67: 50-61.
Williams, P. H. 1998. An annotated checklist of bumble bees with an analysis of patterns of
description (Hymenoptera: Apidae, Bombini). Bulletin of the British Museum of Natural
History (Entomology) 67: 79-152.
Williams, P. H. and J. L. Osborne. 2009. Bumble beevulnerability and conservation world-
wide.Apidologie 40: 367-387.
Williams, P. H., S. A. Cameron, H. M. Hines, B. Cederberg, and P. Rasmont. 2008. A
simplified subgeneric classification of the bumble bees (genus Bombus). Apidologie
39:46-74.
Williams, P., R. Thorp, L. Richardson, and S. Colla. 2014. Bumble Bees of North America.
Princeton, NJ. Princeton University Press.
Winter, K., L. Adams, R. Thorp, D. Inouye, and L. Day. 2006. Importation of non-native bumble
bees into North America: Potential consequences of using Bombus terrestris and other
non-native bumble bees for greenhouse crop pollination in Canada, Mexico and United
States. North American Pollinator Protection Campaign (NAPCC), White Paper.

61
Wolf, S. and R. F. A. Moritz. 2008. Foraging distance in Bombus terrestris (Hymenoptera:
Apidae). Apidologie 38:419-427.
Xerces Society and Thorp. 2010. Petition to List Franklin’s Bumble Bee Bombus franklini
(Frison), 1921, As An Endangered Species Under the U.S. Endangered Species Act.
Submitted by The Xerces Society for Invertebrate Conservation and Dr. Robbin W.
Thorp, June 23, 2010. 40 pp.
Xerces Society for Invertebrate Conservation. 2013. Petition to list the Rusty Patch Bumble
Bee.42 pp.
Yamamoto, I. and J.E. Casida (eds). 1999. Nicotinoid insecticides and nicotinic acetylcholine
receptor. Springer, Berlin Heidelberg New York.
Zayed, A. 2009. Bee genetics and conservation. Apidologie. Springer Verlag. 40 (2): 237-262.
Zayed, A. and L. Packer. 2005. Complementary sex determination substantially increases
extinction proneness of haplodiploid populations. Proceedings of the National Academy
of Sciences 102:10742-10746.
Personal Communications:
Atzet, pers. comm. 2017. Dr. Thomas Atzet, Siskiyou Field Institute, Selma, Oregon, October
27th. 2017.
Brooks, pers. comm. 1997. Melissa Brooks. Unpublished Data. Humboldt State University.
September 16 1997.
Colla, pers. comm. 2018. Sheila Colla. York University. Email response to expert elicitation.
February 17
th
, 2018.
Colyer, pers. comm. 2018. Sheila Colyer, United States Forest Service. Email response to peer
review of draft SSA. January 29th, 2018.
Colyer, pers. comm. 2017. Sheila Colyer, United States Forest Service. Email response to expert
elicitation. November 1
st
, 2017.
Godwin, pers. comm. 2017. Steve Godwin, Bureau of Land Management. Email response to
expert elicitation. November 2
nd
, 2017.
Hatfield, pers. comm. 2017. Rich Hatfield, Senior Endangered Species Conservation Biologist,
Xerces Society, Portland, Oregon. Email response to expert elicitation questionnaire,
October 27
th
, 2017.
Melathopoulos, pers. comm. 2018. Dr. Andony Melathopoulos, Oregon State University,
February 5, 2018.
62
Oregon Department of Agriculture, pers. comm. 2018. Helmuth Rogg and Sarah Kincaid.
Written response to peer review of draft SSA. February 5
th
, 2018.
Schroeder, pers. comm. 2017. Peter Schroeder. Email response to expert elicitation
questionnaire, October 27
th
, 2017.
Thorp, pers. comm. 2017. Dr. Robbin Thorp, Professor Emeritus, University of California, Davis
California. Telephone interview on October 24
th
, 2017 and follow-up email October 27
th
,
2017.
Trail, pers. comm. 2017. Pepper Trail, U.S. Fish and Wildlife Service, Ashland, Oregon. Email
response to expert elicitation questionnaire, October 27
th
, 2017.
United States Department of Agriculture – National Agriculture Statistics Service 2017 pers.
comm. December 29
th
, 2017.
Von Kienast, J. pers. comm. 2018. United States Forest Service, January 29, 2018.

Appendix1:OccurrenceTable‐Table1:KnownoccurrencesofBombusfranklini
Landowner 1923 1925 1930 1946 1949 1950 1952 1953 1958 1963 1964 1968 1969 1976 1980 1986 1988 1989 1990 1992 1994 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017
UnknownLocation unknown 1W,1M
Sutherlin(3miWof) Private
1U
(1)
0(1) 0(1) 0(2) 0(1) 0(2) 0(3) 0(1)
Roseburg Private 1W 2M,1U
Ashland Private 2W 1U 1U
4Q,1W,
1Q
1U
Ashland(8miENE) Private 3W,6M
Ashland(Ashlandpond)
Cityof
Ashland
2W
Ashland(MistletoeRd) Private 0(1) 0(2) 1U(3) 0(4) 0(7) 0(5) 0(2)
Ashland(SOURocaCanyon)
State(no
publicaccess)
2Q
Buncom(Eof) Private
1U
(1)
0(3) 0(1) 0(1)
CentralPoint Private 2W
Copper USFS 7U
Copper(2miN) USFS 2M,2W
Copper(14miN) BLM 1W
Copper(8miWof) USFS 2U
Copper(nr‐see17mi.Wof
Ruch)
USFS
12Q(plus
207other
specimens)
GoldHill Private 1W 3W 1W 7W 12W
GoldHill(3miEof) Private
44U
(4)
0(2)
5U
(7)
0(7) 0(3) 0(4) 0(2) 0(4) 0(2) 0(2)
GrizzleyPeak BLM 1Q 0(2) 0(2) 0(1) 0(2) 0(2) 0(2) 0(2) 0(3) 0(1) 0(2)
Jacksoncampground USFS 2U(2) 0(2) 0(1) 0(1) 0(1)
KenneyMeadows BLM
3U
(2)
0(2) 0(2) 0(2) 0(1) 0(1) 0(1)
LostCreekReservoir Private 1Q 0(1) 0(1) 0(1) 0(1)
Medford Private 8W,10M 2W,1M
2Q,12W,
24M(7)
1M
5W,4
M
Medford(RoxyAnnPeak) Private 2Q
Mt.Ashland USFS
4W
(over2
days)
1Q(plus207
other
specimens)
37U
(3)
19U
(6)
2U
(7)
1U
(5)
19U
(10)
3U
(9)
0(13) 0(11)
1W
(8)
0(7)
Phoenix(Eof) Private 0(1) 0(2)
Ruch Private 6W 12W
3U
(3)
0(2)
1U
(2)
0(1) 0(2) 0(2)
Ruch(SSEof) Private 0(2)
1U
(3)
0(2) 0(1) 0(2) 0(1)
ShaleCityRdtoGrizzley
Peak
Private 1Q
UnionCreek USFS 1Q 0(1)
GrantsPass Private 3W(2)
Merlin Private 1W
Selma(Sof) Private
2U
(1)
0(1) 0(1)
Wonder(Wof)‐reportedas
historicalrecordbutnodate
ofoccurancerecord
reported
Private 0(1)
Hilt Private 1M 1Q 12W
2U
(2)
0(3) 0(3) 0(1) 0(2) 0(1) 0(1) 0(2) 0(2) 0(1)
EverittMem.Hwy USFS 1W
MarbleMtnWilderness
(BackMeadows,southpe
BoulderCreek
USFS ?U
MarbleMtnWildernessBear
Valleyrea5500ft
USFS ?U
MarbleMtnWildernessBig
Meadows^500ft
USFS ?U
MarbleMtnWilderness
LoweWrightLake7000ft
USFS ?U
MarbleMtnWilderness
PacificCrestTrail(.5miS
ParadiseLake)
USFS ?U
MarbleMtnWilderness
UpperKelsyCreek5600ft
USFS ?U
Montegue Private 0(1) 0(1)
Yreka Private 2W
WilloCreek,TrinityAlps,17
miNWeaverville
USFS ?U
TOTAL: 2 2 9 18 3 39 1 9 2 15 5 22 ? 1 1 13 2 15 32 1 2 10 98 2091203 0 0100000000000
CALIFORNIA
SiskiyouCounty
TrinityCounty
YEAR
(Q=Queen,W=worker,M=male,U=individualofunknowntype;numberinparenthesesindicatesnumberofdaysthesitewasvisitedthatyear
)
OREGON
DouglasCounty
JacksonCounty
JosephineCounty
Soilmodificationataportionofthesitein2004.
InundatedbyLakeApplegateaftercompletionofApplegateDaminFallof1980

Printed on 100 percent chlorine-free/100 percent post-consumer content recycled paper
Appendix 2: Dear Interested Party Letter
Reply To: 8185.0153
File Name: DIP Franklin bumble bee.doc
TS Number: 17-576
Dear (Interested Party),
The U.S. Fish and Wildlife Service (Service) is evaluating the status of Franklin’s
Bumble Bee (Bombus franklini) to determine the need for potential listing as an endangered or
threatened species under the Endangered Species Act of 1973, as amended (16 U.S.C 1531 et
seq.; Act). We initiated this process following our receipt of a petition dated June 23, 2010, from
the Xerces Society for Invertebrate Conservation, and Dr. Robbin Thorp (petitioners). The
petitioners requested listing of Franklin’s bumble bee as an endangered species and that critical
habitat be designated for the species. On August 16, 2010, we provided the petitioners with our
determination that an emergency listing was not warranted based on our assessment of the
immediacy of possible threats to Franklin’s bumble bee as presented in the petition. We also
informed the petitioners that at that time, we would not be able to further address the petition due
to requirements to complete a significant number of listing and critical habitat designations. In
September 2011, we published 90-day finding in the Federal Register (76 FR 56381), wherein
we determined that the petition presented substantial information indicating that the listing of the
Franklin’s bumble bee may be warranted. We also requested scientific and commercial data and
other information regarding this species at that time. With this letter, we are providing early
notification to our conservation partners that we are continuing with this status review process
(as initiated with the 90-day finding on September 13, 2011 (76 FR 56381).
Franklin’s bumble bee has been found in an area of about 190 miles north-south and 70
miles east-west in Douglas, Jackson and Josephine counties in southern Oregon and in Siskiyou
and Trinity counties in northern California, which is the most limited distribution known of any
bumble bee species in North America, and perhaps the world. Franklin’s bumble bee is a
eusocial bumble bee, and each colony goes through an annual cycle and only the queen lives
through the winter. The nesting biology of Franklin’s bumble bee is unknown, but like other
Bombus species, it is thought to nest underground in grassy areas, presumably in abandoned
rodent burrows. The flight season of Franklin’s bumble bee is from mid-May, when the queen
emerges from hibernation, to the end of September. Franklin’s bumble bee requires habitat with
a rich supply of floral resources that bloom continuously from spring to autumn. Bumble bees
are generalist foragers, meaning that they gather pollen and nectar from a wide variety of
flowering plants. Unlike honey bees, bumble bees do not produce honey for winter nutrition –
United States Department of the Interior
FISH AND WILDLIFE SERVICE
Oregon Fish and Wildlife Office
2600 SE 98
th
Avenue, Suite 100
Portland, Oregon 97266
Phone: (503) 231-6179 FAX: (503) 231-6195
2
rather, nutrition is derived from nectar, which provides carbohydrates, and from pollen provides
protein.
According to the petition, the primary threats to Franklin’s bumble bee in Oregon and
California, according to the petitioners, include exotic diseases introduced from commercial
bumble bees used for greenhouse pollination of tomatoes and field pollination of a variety of
crops; habitat loss due to destruction, degradation and conversion; pesticides and pollution; and
inadequacy of current rules, regulations and law. The petitioners also identified the following
additional threats: small population size, exotic plant species introduction, increased human use
of native habitat, climate change affecting alpine habitat, and alteration of wildfire severity and
intensity.
Our status review includes consideration of all of the best scientific and commercial data
available to us regarding Franklin’s bumble bee populations, and is not limited to the information
provided in the petition. Over the next several months, we will be gathering and analyzing
available information as part of our evaluation of the species’ status. We are required to use the
best scientific and commercial data available in the development of our finding to ensure our
analysis and finding is as accurate as possible. We are seeking your input to ensure we have the
best scientific data available to inform our finding.
We are particularly seeking information and data for Franklin’s bumble bee throughout
its range in Oregon and California regarding the following:
• Biology, range, and population trends, including:
o Habitat requirements for feeding, breeding, and sheltering;
o Genetics and taxonomy of the population;
o Historical and current range including distribution patterns, and presence or
absence of physical, physiological, or behavioral barriers to movement between
populations;
o Historical and current population levels, and current and projected trends; and
o Past and ongoing conservation measures for the species, its habitat, or both.
• The factors that are the basis for making a listing determination for a species under
section 4(a) of the Act, which are:
o The present or threatened destruction, modification, or curtailment of its habitat or
range;
o Overutilization for commercial, recreational, scientific, or educational purposes;
o Disease or predation;
o The inadequacy of existing regulatory mechanisms; or
o Other natural or manmade factors affecting its continued existence.
While we will accept new information throughout this process, we request that you
provide us with any pertinent information by August 3rd, 2017, to ensure we have adequate time
to consider it during development of our finding. If you have already provided us with any data
recently, thank you; there is no need to resubmit that information, as it will be fully considered in
our status review.
Information should be submitted to Jeff Everett of our Oregon Fish and Wildlife Office at
(503) 231-6952 ([email protected]). Please be aware that all data and information
3
submitted to us, including names and addresses, will become part of the decision record for this
package and will be available for public inspection.
Thank you for your interest in the conservation of Franklin’s bumble bee. If you would
like additional information about the listing process, please contact Rebecca Migala of our
Portland Regional Office at (503) 231-2011 ([email protected]). Additional
information on the listing process is available online at our website at
http://www.fws.gov/endangered/what-we-do/listing-overview.html.
Sincerely,
Paul Henson
State Supervisor

Appendix3:NeonicotinoidPesticideDatabyCounty,1995‐201
5
Imidacloprid Thiamethoxam Clothiandin Imidacloprid Thiamethoxam Clothiandin Imidacloprid Thiamethoxam Clothiandin Imidacloprid Thiamethoxam Clothiandin Imidacloprid Thiamethoxam Clothiandin Imidacloprid Thiamethoxam clothiandin
1995 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 1995 n/a n/a n/a
1996 45.7 0 0 1.5 0 0 2.4 0 0 0 0 0 0 0 0 1996 3.75 0 0
1997 197.5 0 0 0.7 0 0 3 0 0 0 0 0 0 0 0 1997 0.16 0 0
1998 320.5 0 0 0.8 0 0 5.2 0 0 0 0 0 0 0 0 1998 0.239 0 0
1999 1.6 0 0 0.7 0 0 1.2 0 0 0 0 0 0 0 0 1999 n/a n/a n/a
2000 8.8 0 0 3.1 0 0 5.2 0 0 0 0 0 0 0 0 2000 3.05625 0 0
2001 92.8 73.8 0 3.6 0.1 0 7.4 1.2 0 0 0 0 0 0 0 2001 0.374 0 0
2002 18 99 0 6.6 0.2 0 11.3 1.7 0 0 0 0 0 0 0 2002 18.035 0 0
2003 69.3 16 0 0.9 0 0 2.5 0.3 0 14.9 0 0 0 0 0 2003 33.618 0 0
2004 14.5 0 0 4.5 0 0 9.1 0 0 4.6 0 0 0.1 0 0 2004 10.289 0 0
2005 8.7 5.4 0 2.6 0 0 6.2 0.1 0 24.4 0 0 0 0 0 2005 56.928 0 0
2006 2.5 76.1 0 0.7 0.3 0 2.6 1.2 0 13.2 0 0 0 0 0 2006 31.547 0 0
2007 11.4 12 0 2.8 0.1 0 7.9 0.2 0 16.7 2.5 0 0 0 0 2007 39.344 5.427 0
2008 1.6 0.3 0.2 1 0.2 0 2.3 0.1 0 11.7 0 0 0 0 0 2008 26.063 0 0
2009 3.5 54.5 0.2 1.3 0.3 0 4.1 1.1 0 37.1 0 0 0 0 0 2009 64.672 0.08 0
2010 3.1 1.2 0.3 1.1 0.4 0.1 2.3 0.2 0 0 0 0 0 0 0 2010 15.281 19.268 0
2011 9.7 0.3 1 3.3 0.1 0.4 8.2 0.1 0 0 4.8 0 0 0 0 2011 2.219 10.657 0
2012 4.7 11.4 1 2.4 0.1 0.5 5.7 3 0 132.3 3.4 0 0 0 0 2012 305.535 7.498 0
2013 22.4 0.9 3.4 10.6 0.3 1.8 25.2 0.2 0 487.6 14.1 0 0 0 0 2013 473.582 31.156 1.25
2014 152.8 30 3.5 78.8 0.6 1.8 155.6 7.9 0 276.7 12.4 0 0 0 0 2014 685.758 27.37 0
2015 436.8 0 0 37.9 0 0 19.8 0 0 64.1 0 0 0 0 0 2015 160.033 0 0
Imidacloprid Thiamethoxam Clothiandin Imidacloprid Thiamethoxam Clothiandin Imidacloprid Thiamethoxam Clothiandin Imidacloprid Thiamethoxam Clothiandin Imidacloprid Thiamethoxam Clothiandin Imidacloprid Thiamethoxam clothiandin
1995 0.7 0 0 1.8 0 0 0.9 0 0 0 0 0 0 0 0 1995 n/a n/a n/a
1996 2.2 0 0 47.3 0 0 3.9 0 0 0 0 0 0 0 0 1996 0 0 0
1997 1.7 0 0 199.9 0 0 4.6 0 0 0 0 0 0 0 0 1997 0 0 0
1998 1.4 0 0 322 0 0 6 0 0 0 0 0 0 0 0 1998 0 0 0
1999 0.9 0 0 2.2 0 0 1.5 0 0 0 0 0 0 0 0 1999 n/a n/a n/a
2000 2.8 0 0 316.1 0 0 8.7 0 0 0 0 0 0 0 0 2000 3.422 0 0
2001 3.6 0.2 0 92.8 73.9 0 7.4 1.3 0 0 0 0 0 0 0 2001 1.312 0 0
2002 6.7 0.2 0 18.6 99 0 11.5 1.7 0 0 0 0 0 0 0 2002 22.335 0 0
2003 1.4 0.1 0 70.8 16.2 0 3.4 0.4 0 14.9 0 0 0 0 0 2003 0 0 0
2004 4.7 0.3 0 15.3 116.4 0 9.5 1.9 0 4.6 0 0 0.1 0 0 2004 0.23 0 0
2005 2.9 0 0.2 40.4 5.4 0.9 7 0.1 0.9 24.4 0 0 0 0 0 2005 0.738 0 0
2006 0.8 0.3 0.2 2.8 76.1 52.4 2.8 1.2 0.8 13.2 0 0 0 0 0 2006 0.946 0 0
2007 3.6 0.1 1.5 57.6 12 352.7 9.4 0.2 5.2 16.7 2.5 0 0 0 0 2007 1.81 0 0
2008 1.1 0.4 0.3 18.3 49.3 63.4 2.7 0.8 1 11.7 0 0 0 0 0 2008 0.884 0 0
2009 1.4 0.3 0.3 32.5 54.8 50.6 4.7 1.1 0.8 37.1 0 0 0 0 0 2009 1.04 0 0
2010 2.8 0.6 0.1 16.4 45.5 0.3 6.6 1 0 0 0 0 0 0 0 2010 0 0 0
2011 3.7 0.1 0.4 65.1 13.8 1 10.7 0.3 0 0 4.8 0 0 0 0 2011 0.654 0 0
2012 2.8 0.4 0.5 64 68.5 1 8.6 4.1 0 132.3 3.4 0 0 0 0 2012 0.117 0 0
2013 11.2 0.8 1.8 110.7 97.2 9.6 27.6 2.6 0.1 487.6 14.1 0 0 0 0 2013 3.594 0.065 0
2014 79.1 0.9 1.8 215.4 81.4 3.5 157.8 8.8 0 276.7 12.4 0 0 0 0 2014 2.494 0 0
2015 38 0.3 0.1 436.9 47.5 18.8 80.2 0.9 0.3 64.1 0 0 0 0 0 2015 0.362 0 0.02
USGSDatafromhttps://water.usgs.gov/nawqa/pnsp/usage/maps/about.php.
ForallStatesexceptCalifornia,proprietaryfarmsurveypesticide‐usedataareaggregatedandreportedatthemulti‐countyCropReportingDistrictlevel
.
HarvestedcropacreagedatabycountyfromtheUSDepartmentofAgricultureCensusofAgricultureareusedtocalculatethemedianpesticide‐by‐cropuseratesforeachCR
D
EstimatesforCaliforniaareobtainedfromannualDepartmentofPesticideUseReports.MethodsforgeneratingcountylevelpesiticideuseestimatesaredescribedinThelinandStone,2013,andBakerandStone,2015
Thesedataareestimates‐pleaserefertotheUSGSwebsiteformoredetailedinformationonhowtheestimatesaregeneratedandimportantlimitationsondataus
e
CaliforniaPesticideInformationPortalcanbefoundathttp://calpip.cdpr.ca.go
v
CalPipdatabasedoesnothaveresultsfor1995and1999
.
2013rawCalpipdataincludesasingleapplicationof620.423poundsofimidaclopridon44acresofpotatosinsiskiyoucounty;notincludedhereassuspectederroneousdat
a
SiskiyouCounty,CACalPiPData
TrinityCounty,CACalPiPData
EPEST_LOWallcounties
EPEST_HIGHallcounties
TrinityCounty,CASiskiyouCounty,CADouglasCounty,ORJacksonCounty,ORJosephineCounty,OR
JosephineCounty,OR JacksonCounty,OR DouglasCounty,OR SiskiyouCounty,CA TrinityCounty,CA
Appendix 4: Expert Elicitation Questionnaire
Franklin’s bumble bee (Bombus franklini)- Questions from USFWS 10/27/2017
In our status assessment of B. franklini, we need to articulate the habitat requirements for a viable population of the species; we look at
viability in terms of what a reasonable naturalist would consider a stable, self-sustaining population. In order to get a better understanding of
the species’ population structure and habitat requirements, as well as the factors that might have influenced B. franklini populations, we have
the following questions:
1. What would the number of individual B. franklini recorded at a site indicate?
• Could you draw any conclusions on the number of colonies represented at the site?
(e.g. 3 bees= 1 colony, or 44 bees= more than 1 colony?)
• How big is the typical (most likely) colony foraging area (in km
2
) of B. franklini or other Bombus species that have similar foraging
behavior?
What is the largest?
Smallest?
• What is the probability (high, medium, or low) that Bombus colonies overlap in their foraging areas? (0-32% = low, 33-65% = medium, 66-
100% = high)
• What is the probability (high, medium, low) that at sites where multiple bees were counted over several visits (in all historical records
and surveys), that the same individual bee might have been counted twice? (0-32% = low, 33-65% = medium, 66-100% = high)
2. How many colonies would make up a viable population (as defined above in bold) of B. franklini or a similar species?
How large (in km
2
) would a habitat patch have to be to support a viable population?

3. In the past (pre- 1998), when people went out to collect native bumble bees including B. franklini, what was the probability (high, medium,
low) that a specimen of B. franklini was included in the day's collection? (0-32% = low, 33-65% = medium, 66-100% = high)
• Could people usually collect a Franklin's specimen if they knew where to find them or was it always a challenge to find them even at
sites where they previously occurred?
4. Can we infer from the historical data that a higher count of B. Franklini at a site was due to higher abundance of bees? Or… should we not
infer this because of the varying intensity of survey efforts (i.e. maybe they found more bees that year because they looked harder for the
bees)?
5. Can you suggest a species of Bombus that is similar to B. franklini that has been studied more (a species we can use as a surrogate for
information on population structure and habitat requirements)? If so, are there any caveats or considerations we should keep in mind
when using this species as a surrogate?
6. Would you consider any of the sites where B. franklini were found in the past (Table 1 below) extirpated or no longer viable?
Why or why not? How confident are you in your answer? (highly confident, confident, minimally confident)
7. In Table 1, please fill in any site-specific information you have on the following potential stressors at last known occurrence sites of B.
franklini. Do you think there are any other factors that may have led to the decline of B. franklini at any of these sites or elsewhere in the
historic range (Douglas, Jackson, and Josephine Counties, Oregon, and Siskiyou and Trinity Counties, California)?

Table 1: Potential stressors at known occurrence sites of Bombus franklini from 1997-2006 (occurrence site information taken from 2010 Petition
to List Franklin’s Bumble Bee (Thorp et al. 2010, p. 9 and Appendix 1)).
Pesticide Use
Grazing
Proximity to
commercially raised
bees
Wildfire
Changes in bloom time
of forage Vegetation
Invasive Species
Changes in habitat
(development or other)
Jackson County, Oregon
Sutherlin (3 miles
West of)
Ashland
Ashland Pond
Ashland (SOU_
Roca Canyon)
Buncom (1.5 miles
East of)
Gold Hill (3 miles
East of)
Significant excavation
and deposited soil in
2004- altered 50% of
bumble bee habitat
Grizzley Peak/ Shale
City Road
Jackson
campground
Kenney Meadows
Lost Creek
Reservoir
Medford- Roxy Ann
Peak
Mt. Ashland
Pesticide Use
Grazing
Proximity to
commercially raised
bees
Wildfire
Changes in bloom time
of forage Vegetation
Invasive Species
Changes in habitat
(development or other)
Phoenix (E of)
Ruch
Ruch (4 miles SSE
of)
Josephine County, Oregon
Selma (South of)
Siskiyou County, California
Hilt
8. In looking at the draft distribution map of known occurrences of B. franklini (below), are there areas in Douglas, Jackson, Josephine,
Siskiyou, and Trinity Counties in addition to these occurrence sites that might contain its known foraging plants (and therefore potential B.
franklini habitat): lupine (Lupinus spp.), California poppy (Eschscholzia californica), horsemint or nettle-leaf giant hyssop (Agastache
urticifolia) and mountain monardella (Monardella odoratissima)?

